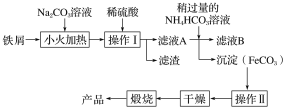
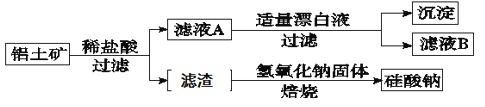
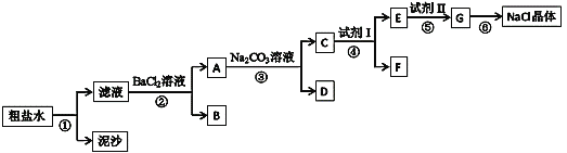
��Ŀ����
����Ŀ����1����16gO2����ԭ��������ͬ��NH3��״���������________L��
��2����֪2LAl2(SO4)3��Һ��c(Al3+)=3mol/L����c(SO42��)=__________����3L__________mol/LNa2SO4��SO42�������ʵ���Ũ����ȡ�
��3��ͬ��ͬѹ�£�ͬ�����NH3��H2S�����������Ϊ_____________��ͬ������NH3��H2S����������Ϊ___________����������ԭ������ȣ����ǵ������Ϊ____________��
��4���ڱ�״���£�8.96L��CH4��CO�Ļ�����壬����������������ܶ���9.5���������ƽ��Ħ������Ϊ__________�����������CH4�����Ϊ__________��һ����̼����������Ϊ__________������ԭ�Ӹ�����Ϊ_______________��
���𰸡�5.6 4.5mol/L 4.5 1:2 2:1 2:3 19g/mol 6.72L 36.8% 15:2
��������
��1��16gO2�����ʵ���=![]() ����ԭ�ӵ�����=0.5mol��2��NA= NA��һ��NH3���Ӻ���4��ԭ�ӣ�����NAԭ�ӵ�NH3�ķ�����ĿΪ
����ԭ�ӵ�����=0.5mol��2��NA= NA��һ��NH3���Ӻ���4��ԭ�ӣ�����NAԭ�ӵ�NH3�ķ�����ĿΪ![]() ��NH3���ʵ���=
��NH3���ʵ���=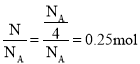 ����״���������0.25mol��22.4L/mol=5.6L��
����״���������0.25mol��22.4L/mol=5.6L��
����5.6��
��2����֪2LAl2(SO4)3��Һ��c(Al3+)=3mol/L����c(SO42��)=![]() ��c(Al3+)=
��c(Al3+)=![]() ��3mol/L =4.5mol/L�� ʹNa2SO4��SO42�������ʵ���Ũ��ҲΪ4.5mol/L����Na2SO4��Һ��Ũ��Ϊ4.5mol/L������������Һ������أ�
��3mol/L =4.5mol/L�� ʹNa2SO4��SO42�������ʵ���Ũ��ҲΪ4.5mol/L����Na2SO4��Һ��Ũ��Ϊ4.5mol/L������������Һ������أ�
����4.5��
��3��ͬ��ͬѹ�£����֮�ȵ������ʵ���֮�ȣ������ͬ�����ʵ�����ͬ����ֱ�Ϊ1mol��NH3��H2S�����������=Ħ������֮��=17g/mol:34g/mol=1:2���ֱ�Ϊ17g��NH3��H2S����������=���ʵ���֮��=![]() =2:1����������ԭ������ȣ���NH3��H2S�ֱ���NA����ԭ�ӣ���NH3�����ʵ���=
=2:1����������ԭ������ȣ���NH3��H2S�ֱ���NA����ԭ�ӣ���NH3�����ʵ���=![]() mol��H2S�����ʵ���=
mol��H2S�����ʵ���=![]() mol�����ǵ������=���ʵ���֮��=
mol�����ǵ������=���ʵ���֮��=![]() mol ��
mol ��![]() mol =2:3��
mol =2:3��
����1:2�� 2:1��2:3��
��4���ڱ�״���£�8.96L��CH4��CO�Ļ����������ʵ���=![]() =0.4mol������������������ܶ���9.5���������ƽ��Ħ������Ϊ9.5��2g/mol=19g/mol������������CH4��CO�����ʵ����ֱ�Ϊx��y����x+y=0.4mol��16g/mol��x+28g/mol��y=0.4mol ��19g/mol �����ã�x=0.3mol��y=0.1mol������£�0.3mol CH4�����Ϊ0.3mol��22.4L/mol=6.72L��һ����̼����������Ϊ
=0.4mol������������������ܶ���9.5���������ƽ��Ħ������Ϊ9.5��2g/mol=19g/mol������������CH4��CO�����ʵ����ֱ�Ϊx��y����x+y=0.4mol��16g/mol��x+28g/mol��y=0.4mol ��19g/mol �����ã�x=0.3mol��y=0.1mol������£�0.3mol CH4�����Ϊ0.3mol��22.4L/mol=6.72L��һ����̼����������Ϊ![]() =36.8%��0.3mol CH4���е�ԭ����ĿΪ0.3mol��5��NA=1.5NA��0.1mol CO���е�ԭ����ĿΪ0.1mol��2��NA=0.2NA������ԭ�Ӹ�����Ϊ1.5NA��0.2NA=15:2��
=36.8%��0.3mol CH4���е�ԭ����ĿΪ0.3mol��5��NA=1.5NA��0.1mol CO���е�ԭ����ĿΪ0.1mol��2��NA=0.2NA������ԭ�Ӹ�����Ϊ1.5NA��0.2NA=15:2��
����15:2��

 Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д� ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�