��Ŀ����
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
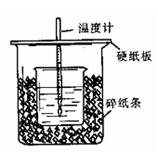
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ��������� ��
��2���ձ���������ֽ���������� ��
��3��Ҫ�ظ���������ʵ���Ŀ���� ��
��4�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ��ƫС����Ӱ�족)�������µ���10��ʱ���У���ʵ��������ɽϴ������ԭ����_______ ��
��5�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)�������к��� (���ȡ�����ȡ�)���������� ��
��6������ͬŨ�Ⱥ�����Ĵ��ᣨCH3COOH������HCl��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �����ƫ��ƫС����Ӱ�족����
��7������ƽ�в�������õ��������£�
������HCl��NaOH��Һ���ܶȶ�����Ϊ1g/cm3���кͺ����ɵ���Һ�ı�����C="4.18" J/��g���棩����ʵ���õ��к���Ϊ ��

��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ��������� ��
��2���ձ���������ֽ���������� ��
��3��Ҫ�ظ���������ʵ���Ŀ���� ��
��4�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ��ƫС����Ӱ�족)�������µ���10��ʱ���У���ʵ��������ɽϴ������ԭ����_______ ��
��5�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)�������к��� (���ȡ�����ȡ�)���������� ��
��6������ͬŨ�Ⱥ�����Ĵ��ᣨCH3COOH������HCl��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �����ƫ��ƫС����Ӱ�족����
��7������ƽ�в�������õ��������£�
| �¶� ��� | ��ʼ�¶�t1/�� | ��ֹ�¶� T2/�� | �¶Ȳ� ��t/�� | ||
| HCl | NaOH | ƽ��ֵ | |||
| 1 | 25 | 25 | | 27.3 | |
| 2 | 25 | 25 | | 27.4 | |
| 3 | 25 | 25 | | 28.6 | |
��1������������ (2��)
��2�����ȱ��¡�����ʵ���������������ʧ ��2�֣�
��3����β�����ƽ��ֵ���Լ���ʵ����� ��2�֣�
��4��ƫС��1�֣������½ϵ�ʱ��Ӧ��ϵ��ɢ�ȱȽϿ죬������ʧ���ƫ�ͣ�1�֣�
��5������ȣ�1�֣�����ȣ�1�֣����к�����ָ�������кͷ�Ӧ����1molH2Oʱ���ų������������ᡢ��������أ�2�֣�
��6��ƫС��1�֣�
��7��39.3kJ/mol ��H= -39.3kJ/mol�� ��2�֣�
��2�����ȱ��¡�����ʵ���������������ʧ ��2�֣�
��3����β�����ƽ��ֵ���Լ���ʵ����� ��2�֣�
��4��ƫС��1�֣������½ϵ�ʱ��Ӧ��ϵ��ɢ�ȱȽϿ죬������ʧ���ƫ�ͣ�1�֣�
��5������ȣ�1�֣�����ȣ�1�֣����к�����ָ�������кͷ�Ӧ����1molH2Oʱ���ų������������ᡢ��������أ�2�֣�
��6��ƫС��1�֣�
��7��39.3kJ/mol ��H= -39.3kJ/mol�� ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ�����ݻ�Ϊ2 L���ܱ������г���10 mol N2��5 mol O2���ﵽƽ���NO�����ʵ���Ϊ2 mol����T ��ʱ�÷�Ӧ��ƽ�ⳣ��K= �� ��������������С�������λ���֣�
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ�����ݻ�Ϊ2 L���ܱ������г���10 mol N2��5 mol O2���ﵽƽ���NO�����ʵ���Ϊ2 mol����T ��ʱ�÷�Ӧ��ƽ�ⳣ��K= �� ��������������С�������λ���֣� 
 ��˸�ͬѧ�ó����ۡ�ú̿ȼ��ʱ������ˮ����ʹú̿ȼ�շų������������������֪ú̿��ȼ����Ϊ��393.15 kJ/mol��������ȼ����Ϊ��242 kJ/mol��һ����̼��ȼ����Ϊ��283 kJ/mol��
��˸�ͬѧ�ó����ۡ�ú̿ȼ��ʱ������ˮ����ʹú̿ȼ�շų������������������֪ú̿��ȼ����Ϊ��393.15 kJ/mol��������ȼ����Ϊ��242 kJ/mol��һ����̼��ȼ����Ϊ��283 kJ/mol��



 ��
�� ʱ��
ʱ�� ��ȫȼ��������̬ˮ���ų�
��ȫȼ��������̬ˮ���ų� ��������������ȼ����Ϊ241.8
��������������ȼ����Ϊ241.8


 ��
�� ������˵����ȷ���ǣ� ��
������˵����ȷ���ǣ� ��
 ���ӣ�������
���ӣ�������
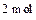 Һ̬ˮ����
Һ̬ˮ����
 ̼�����õ���ʱ����ų�������Ϊ
̼�����õ���ʱ����ų�������Ϊ

 HBr+H�������Է�Ӧ���̵�ʾ��ͼ��������������ȷ����
HBr+H�������Է�Ӧ���̵�ʾ��ͼ��������������ȷ����