��Ŀ����
���Ļ������ж�������+6��Cr��ǿ�����ԣ��䶾����+3��Cr���Ե�100������ˣ�����Ժ����ķ�ˮ���д�����Ŀǰ�о��Ͳ��õĴ���������Ҫ�У�
����һ����ԭ����
���������Խ�������FeSO4��NaHSO3�Ƚ�+6��Cr��ԭ��+3��Cr�������������£�
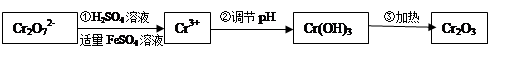
��ش��������⣺
��1���������̢ٷ�����Ӧ�����ӷ���ʽ�� ��
��2�����ڢ� ʹFeSO4�ʵ������������������ɲ������д��ԡ���������������壨Fe3O4��FeO��Fe2O3���ĸ��������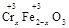 ��y
��y O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol FeSO4��7H2O�������
O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol FeSO4��7H2O������� ��y
��y O����ѧʽ�� ��
O����ѧʽ�� ��
�����о����֣�����������ԭ���������Գ�ȥCr6+�����ܳ�ȥ��ˮ�е�����Mn2+�����о���м������pHֵ�Է�ˮ�и�����ȥ���ʵ�Ӱ�죬
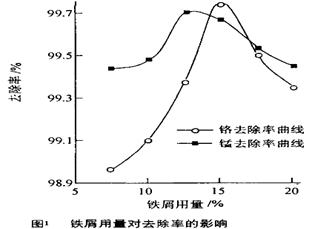
��3��ȡ100mL��ˮ��250 mL����ƿ�У�����pHֵ���涨ֵ���ֱ���벻ͬ���ķ���м���õ���м�����Ը�����ȥ���ʵ�Ӱ������ͼ1��ʾ������pHһ��ʱ����ˮ����м����Ϊ ʱ�̡���ȥ�������
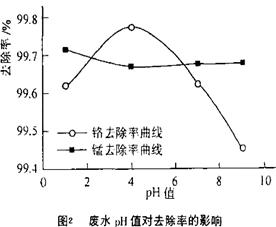
��4��ȡ100mL��ˮ��250 mL����ƿ�У�����涨�������ۣ����ɲ�ͬ��pHֵ���õ�pHֵ�Ը�����ȥ���ʵ�Ӱ������ͼ2��ʾ��������м����һ��ʱ����ˮpH= ʱ�̡���ȥ�������
����������ⷨ������+6��Cr�ķ�ˮ��������ڣ������������������������Ȼ��ƽ��е�⣺���������ɵ�Fe2+��Cr2O72-������Ӧ�����ɵ�Fe3+��Cr3ʮ����������OHһ�������Fe��OH��3��Cr��OH��3������ȥ��
��5��д����������Ӧ�ĵ缫����ʽ ��
��6�������Ϸ�����1��104 L������+6�ۣ�78 mg / L�ķ�ˮ�����ʱ�����������ĵ���������Ϊ________kg��
����һ����ԭ����
���������Խ�������FeSO4��NaHSO3�Ƚ�+6��Cr��ԭ��+3��Cr�������������£�
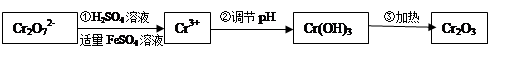
��ش��������⣺
��1���������̢ٷ�����Ӧ�����ӷ���ʽ�� ��
��2�����ڢ� ʹFeSO4�ʵ������������������ɲ������д��ԡ���������������壨Fe3O4��FeO��Fe2O3���ĸ��������
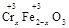 ��y
��y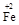 O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol FeSO4��7H2O�������
O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol FeSO4��7H2O�������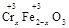 ��y
��y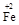 O����ѧʽ�� ��
O����ѧʽ�� �������о����֣�����������ԭ���������Գ�ȥCr6+�����ܳ�ȥ��ˮ�е�����Mn2+�����о���м������pHֵ�Է�ˮ�и�����ȥ���ʵ�Ӱ�죬
��3��ȡ100mL��ˮ��250 mL����ƿ�У�����pHֵ���涨ֵ���ֱ���벻ͬ���ķ���м���õ���м�����Ը�����ȥ���ʵ�Ӱ������ͼ1��ʾ������pHһ��ʱ����ˮ����м����Ϊ ʱ�̡���ȥ�������
��4��ȡ100mL��ˮ��250 mL����ƿ�У�����涨�������ۣ����ɲ�ͬ��pHֵ���õ�pHֵ�Ը�����ȥ���ʵ�Ӱ������ͼ2��ʾ��������м����һ��ʱ����ˮpH= ʱ�̡���ȥ�������


����������ⷨ������+6��Cr�ķ�ˮ��������ڣ������������������������Ȼ��ƽ��е�⣺���������ɵ�Fe2+��Cr2O72-������Ӧ�����ɵ�Fe3+��Cr3ʮ����������OHһ�������Fe��OH��3��Cr��OH��3������ȥ��
��5��д����������Ӧ�ĵ缫����ʽ ��
��6�������Ϸ�����1��104 L������+6�ۣ�78 mg / L�ķ�ˮ�����ʱ�����������ĵ���������Ϊ________kg��
��16�֣���1��Cr2O72-��14H+��6Fe2+=6Fe3+��2Cr3ʮ��7H2O��2�֣���
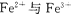
��2��ʹ��Һ�еı���ǡ�������ֹFe2+�����������������Ը��֣���2�֣�
�ô���������2�֣� Cr0.5Fe1.5O3��FeO��2�֣�
��3��15% ��2�֣���4�� 4 ��2�֣���5��2H2O��2e����H2����2OH���� ��2�֣� ��6�� 2��52 ��2�֣�
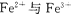
��2��ʹ��Һ�еı���ǡ�������ֹFe2+�����������������Ը��֣���2�֣�
�ô���������2�֣� Cr0.5Fe1.5O3��FeO��2�֣�
��3��15% ��2�֣���4�� 4 ��2�֣���5��2H2O��2e����H2����2OH���� ��2�֣� ��6�� 2��52 ��2�֣�
�����������1��Cr2O72���н�ǿ�����ԣ�FeSO4��Fe2+��һ���Ļ�ԭ�ԣ������Խ����з���������ԭ��Ӧ����ʵ�����̿�֪���ڢٲ���Ӧ��Cr2O72�������������½�Fe2+����ΪFe3+����������ԭΪCr3+�������غ�Ԫ���غ㼰����������֪����Ӧ��ˮ���ɣ���Ӧ���ӷ���ʽΪCr2O72��+14H++6Fe2+=2Cr3++6Fe3++7H2O��
��2�������к������������������������Fe2+����������������ʹ��Һ��Fe2����Fe3���ı�����ǡ����������������д��ԣ�����ʹ�������������ϼ��ķ������ô������������ݷ�Ӧ�����ӷ���ʽ��֪��1mol Cr2O72��������6mol�������ӣ�ͬʱ����2mol Cr3ʮ����ʣ���������ӵ����ʵ�����4mol�����Ը���ԭ���غ�ͻ��ϼ۴�����Ϊ0��֪��
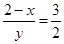 ��3x��6��3x��2y��8�����x��0.5��y��1�����Ը���������Ļ�ѧʽ��Cr0.5Fe1.5O3��FeO��
��3x��6��3x��2y��8�����x��0.5��y��1�����Ը���������Ļ�ѧʽ��Cr0.5Fe1.5O3��FeO����3������ͼ1֪����������������ʱ�����ӵ�ȥ������������С������ˮ����м����Ϊ15%ʱ�̡���ȥ������á�
��4������ͼ2֪������Һ��pHֵ������ʱ�������ӵ�ȥ�����ȼ�С��������ȥ������������С����pH=4ʱ�̡���ȥ������á�
��5�������������õ����ӣ���������ˮ������������ӵõ����ӣ�����������Ӧ�ĵ缫����ʽ2H2O��2e����H2����2OH����
��6��1��104 L������+6�ۣ�78 mg / L�ķ�ˮ�и�Ԫ�ص�������78 mg / L��10000��780000mg��780g�����ʵ�����780g��52g/mol��15mol���ڷ�Ӧ�еõ�15mo��3��45mol���ӣ���˸��ݵ��ӵĵ�ʧ�غ��֪���μӷ�Ӧ������������45mol����������������������������45mol��56g/mol��2520g��2.52kg��
�����������Թ�ҵ��ˮ����Ϊ���壬����������ԭ��Ӧ�����ӷ�Ӧ���缫��Ӧʽ����д�Լ��йؼ���ȣ��Ѷ��еȣ��ؼ�����ʵ����������������ԭ��Ӧ�жϷ��������ӷ�Ӧ���Ƕ�ѧ���ۺ������Ŀ��飮��һ�����������������⣬����������ѧ���������������ͷ�ɢ˼ά������Ҳ����������ѧ���Ļ���������ʶ������ѧ����ѧ��������

��ϰ��ϵ�д�
 һ����������ϵ�д�
һ����������ϵ�д�
�����Ŀ
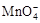 ����C2O42?�����ӷ���ʽ����������
����C2O42?�����ӷ���ʽ����������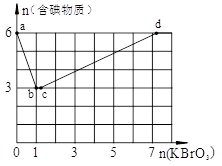
 Cu (HDZ)2+2H+���ټ���CCl4��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
Cu (HDZ)2+2H+���ټ���CCl4��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
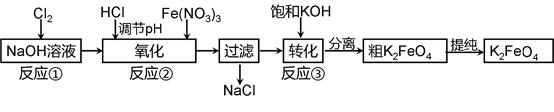
 4Fe(OH)3����8OH��+3O2��,��K2FeO4������ˮ�����е������� ��
4Fe(OH)3����8OH��+3O2��,��K2FeO4������ˮ�����е������� ��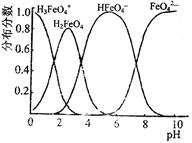
 Na++H++SO42һ
Na++H++SO42һ ��Na+��Cl-����Na2O2��HCl��Al2O3�����ʵ���֮����Ϊ�� ��
��Na+��Cl-����Na2O2��HCl��Al2O3�����ʵ���֮����Ϊ�� ��