��Ŀ����
��ˮ��������ʱ������˫����(H2Dz����Ԫ����)�ѽ���������ϳɵ����Ե����ʣ�����CCl4��ȡ�����Ӷ��ѽ������Ӵ�ˮ��Һ����ȫ�������������˫����(H2Dz)��CCl4������ˮ�е�Cu2+ʱ���ȷ�����Ϸ�Ӧ��Cu2++2H2DZ Cu (HDZ)2+2H+���ټ���CCl4��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
Cu (HDZ)2+2H+���ټ���CCl4��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
��1��д��˫�����Fe3+��ϵ����ӷ���ʽ��_____________________����ȡFe3+�Ĺ�����Ҫ�������˵���ȣ������Һ��pH����������___________________________��
��2����ͼ����˫����(HzDz)��CCl4�����ȡijЩ�������ӵ�������ߣ�����ӳ����ȡijЩ��������ʱ���˵�pH��Χ��E����ʾij�ֽ����������������ʽ��ȡ����İٷ��ʡ�
ij��ҵ��ˮ�к���Hg2+��Bi3+��Zn2+����˫���꣨H2Dz��~ CCl4�����ȡ��������ˮ��
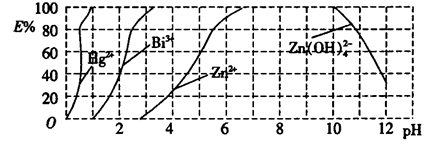
������ȫ����ˮ�е�Hg2+����������������Һ��pH=________��
�ڵ�����pH=2ʱ����(Bi)�Ĵ�����ʽ��_________________��
��3����ˮ�е��ǹ�����(Hg2+ 2)����ת���ɹ�����(Hg2+)������˫������ϡ�ij������ˮ�к��н϶���Ȼ��ǹ�(Hg2Cl2)������������(K2S2O8)������(Hg2+ 2)��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________��
 Cu (HDZ)2+2H+���ټ���CCl4��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
Cu (HDZ)2+2H+���ټ���CCl4��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С���1��д��˫�����Fe3+��ϵ����ӷ���ʽ��_____________________����ȡFe3+�Ĺ�����Ҫ�������˵���ȣ������Һ��pH����������___________________________��
��2����ͼ����˫����(HzDz)��CCl4�����ȡijЩ�������ӵ�������ߣ�����ӳ����ȡijЩ��������ʱ���˵�pH��Χ��E����ʾij�ֽ����������������ʽ��ȡ����İٷ��ʡ�
ij��ҵ��ˮ�к���Hg2+��Bi3+��Zn2+����˫���꣨H2Dz��~ CCl4�����ȡ��������ˮ��

������ȫ����ˮ�е�Hg2+����������������Һ��pH=________��
�ڵ�����pH=2ʱ����(Bi)�Ĵ�����ʽ��_________________��
��3����ˮ�е��ǹ�����(Hg2+ 2)����ת���ɹ�����(Hg2+)������˫������ϡ�ij������ˮ�к��н϶���Ȼ��ǹ�(Hg2Cl2)������������(K2S2O8)������(Hg2+ 2)��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________��
��1��Fe3++3H2DZ
 Fe(HDZ)3+3H+ (2��) ��Fe3+���γ�Fe��OH��3����(1��)
Fe(HDZ)3+3H+ (2��) ��Fe3+���γ�Fe��OH��3����(1��)��2����1(1��)��Bi3+��Bi(HDZ)3(2��)
��3��Hg2Cl2+K2S2O3 = 2HgSO4��+2 KCl(2��)
���������
��1����Ϸ�Ӧ��Cu2++2H2DZ
 Cu (HDZ)2+2H+���ɵ�Fe3++3H2DZ
Cu (HDZ)2+2H+���ɵ�Fe3++3H2DZ Fe(HDZ)3+3H+�������Һ��pH����������Fe3+���γ�Fe��OH��3�������ò���Fe(HDZ)3��
Fe(HDZ)3+3H+�������Һ��pH����������Fe3+���γ�Fe��OH��3�������ò���Fe(HDZ)3����2���ٹ۲�ͼƬ�������ɵ��������Һ��pH=1������pH=2ʱ����(Bi)��������Bi(HDZ)3��������ʽ��Bi3+��Bi(HDZ)3��
��3�����ݻ��ϼ۱仯�ɵ�Hg2Cl2+K2S2O3 = 2HgSO4��+2 KCl��

��ϰ��ϵ�д�
�����Ŀ

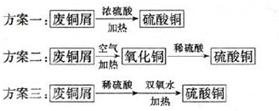
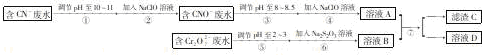
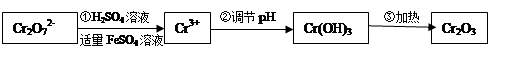
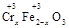 ��y
��y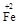 O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol FeSO4��7H2O�������
O��(ע��X�����Ƿ���)�����Ϊ��������ƿ���������Ŀ���� ��ʹ�������������ϼ��ķ����� �����账����1 mol Cr2O72���ķ�ˮ������Ҫ����10mol FeSO4��7H2O�������


 I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�
I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�