题目内容
已知A、B、C、D、E都是元素周期表中的前20号元素,它们的原子序数依次增大。B、C、D同周期,A、D同主族,B,C、D的最高价氧化物的水化物两两混合均能发生反应生成盐和水。E元素的原子核外共有20种不同运动状态的电子,且E的原子序数比D大4。
(1)B、C的第一电离能较大的是____________________(填元素符号)。
(2)A的氢化物的分子空间构型为____________,其中心原子采取____________杂化。
(3)A和D的氢化物中,沸点较高的是_____________(填化学式),其原因是______________________________________。
(4)仅由A与B元素组成,且含有非极性键的化合物是________________(填化学式)。
(5)E的核外电子排布式是___________________。
(6)B的最高价氧化物对应的水化物,其溶液与C单质反应的化学方程式是_________________ 。
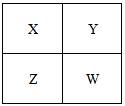
(7)E单质在A单质中燃烧时得到一种白色晶体,其晶体的晶胞结构如右图所示,则该晶体的化学式为___________________。
(1)B、C的第一电离能较大的是____________________(填元素符号)。
(2)A的氢化物的分子空间构型为____________,其中心原子采取____________杂化。
(3)A和D的氢化物中,沸点较高的是_____________(填化学式),其原因是______________________________________。
(4)仅由A与B元素组成,且含有非极性键的化合物是________________(填化学式)。
(5)E的核外电子排布式是___________________。
(6)B的最高价氧化物对应的水化物,其溶液与C单质反应的化学方程式是_________________ 。
(7)E单质在A单质中燃烧时得到一种白色晶体,其晶体的晶胞结构如右图所示,则该晶体的化学式为___________________。

(1)Al (2分)
(2)V型或折线型 (1分) sp3 (1分)
(3)H2O (1分) 水分子之间可以形成氢键,而H2S分子之间不能形成氢键。(2分)
(4)Na2O2 (2分)
(5)1S22S22P63S23P64S2 (2分)
(6)2Al + 2NaOH + 6H2O = 2Na[Al(OH)4] + 3H2↑或2Al + 2NaOH + 2H2O = 2NaAlO2+ 3H2↑(2分)
(7)CaO2 (2分)
(2)V型或折线型 (1分) sp3 (1分)
(3)H2O (1分) 水分子之间可以形成氢键,而H2S分子之间不能形成氢键。(2分)
(4)Na2O2 (2分)
(5)1S22S22P63S23P64S2 (2分)
(6)2Al + 2NaOH + 6H2O = 2Na[Al(OH)4] + 3H2↑或2Al + 2NaOH + 2H2O = 2NaAlO2+ 3H2↑(2分)
(7)CaO2 (2分)
略

练习册系列答案
相关题目
 小的元素是 ,原子半径最大的元素是
小的元素是 ,原子半径最大的元素是  单质是
单质是 
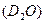 A和普通水
A和普通水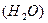 B分别跟足量的金属钠反应,下列叙述正确的是( )。
B分别跟足量的金属钠反应,下列叙述正确的是( )。