��Ŀ����
����Ŀ����ͼ��ij�о���ѧϰС����ƵĶ�һ�ַϾɺϽ���ɷ�(����Cu��Fe��Si���ֳɷ�)���з��롢���������õĹ�ҵ���̣�ͨ���������ܵõ����õĵ��ʡ�����(��ʽ�������ľۺ���)������(Fe2O3)���̷�(FeSO4��7H2O)��
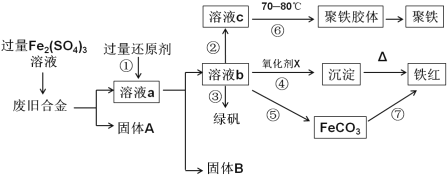
(1)���й����Ļ�ԭ��Ӧ��____________________��
(2) ����Һb�м�������KMnO4��Һ������Ӧ�����ӷ���ʽΪ_______________________��
(3)���м���H2O2��������pHֵ�õ���Һc�������У�����Һc���Ƶ�70-80����Ŀ����________��
(4)�����У�����Ũ����Ҫ�Ĺ������������ƾ����⣬����_________________________��
(5)�����������X��________________________��
(6)�����У�����Һb������NH4HCO3��Һ���õ�FeCO3������д�����ӷ�Ӧ����ʽ ______________��
���𰸡����� MnO4-+5Fe2+ +8H+=Mn2+ +5Fe3+ +4H2O �����¶ȴٽ�Fe3+��ˮ�⣬�����¶Ȳ����ھ������γ�(������ȫˮ��) ���������� Na2O2��NaClO Fe2+ +2HCO3- =FeCO3 ��+ CO2 ��+H2O
��������
��������ͼ�����������Fe2(SO4)3��Һ����Fe��Cu��Ӧ������AΪSi��a�к���Fe2+��Fe3+��Cu2+�����뻹ԭ����b��Һ��ֻ����Fe2+�����Ԣ��й����Ļ�ԭ��Ӧ�����ۣ�����B��ͭ������Һb�м���H2O2����Fe2+����ΪFe3+��Fe3+��ˮ�����ڢ��У�����Һc���Ƶ�70��80���Ŀ���������¶ȴٽ�Fe3+��ˮ�⣬�����¶Ȳ����ھ������γɣ�����Һ����������Һ�м�����NH4HCO3��Һ���õ�FeCO3������FeCO3�����������������ݴ˴��⡣
��1����������ͼ����Һa�к���Fe2+��Fe3+��Cu2+�����뻹ԭ����b��Һ��ֻ����Fe2+�����Ԣ��й����Ļ�ԭ��Ӧ�������ʴ�Ϊ�����ۡ�
��2����Һb�к���Fe2+����������KMnO4��Һ������Ӧ�����ӷ���ʽΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O���ʴ�Ϊ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
��3����Һb�к���Fe2+����b�м���H2O2����Fe2+����ΪFe3+��Fe3+��ˮ�����ڢ��У�����Һc���Ƶ�70��80���Ŀ���������¶ȴٽ�Fe3+��ˮ�⣬�����¶Ȳ����ھ������γɣ��ʴ�Ϊ�������¶ȴٽ�Fe3+��ˮ�⣬�����¶Ȳ����ھ������γ�(������ȫˮ��)��
��4������Ũ����Ҫ�Ĺ������������ƾ����⣬�����������������ʴ�Ϊ��������������
��5����������ͼ���ڢ��е�������X��Na2O2��NaClO���ʴ�Ϊ��Na2O2��NaClO��
��6������Һ����������Һ�м�����NH4HCO3��Һ���õ�FeCO3�����������ӷ�Ӧ����ʽFe2++2HCO3-=FeCO3��+CO2��+H2O���ʴ�Ϊ��Fe2++2HCO3-=FeCO3��+CO2��+H2O��

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�����Ŀ�����ڿ��淴ӦN2��g��+3H2��g��![]() 2NH3��g����H��0�������о�Ŀ�ĺ�ʾ��ͼ�������
2NH3��g����H��0�������о�Ŀ�ĺ�ʾ��ͼ�������
A | B | C | D | |
�о�Ŀ�� | ѹǿ�Է�Ӧ��Ӱ��P2��P1 | �¶ȶԷ�Ӧ��Ӱ�� | ƽ����ϵ���ӵ����Է�Ӧ��Ӱ�� | �����Է�Ӧ��Ӱ�� |
ͼʾ |
|
|
|
|
A. AB. BC. CD. D








