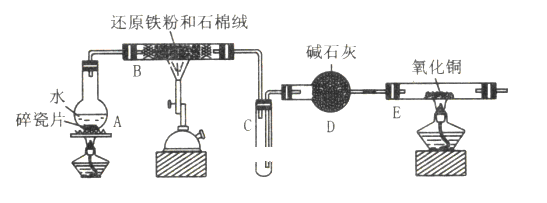
��Ŀ����
����Ŀ������������һ�ֳ���ʳƷ���Ӽ���Ϊ���ijʳƷ���������κ���(ͨ����1 kg��Ʒ�к�SO2��������)��ij�о�С���������������ʵ�����̣�
��1������������������H2O2��______�ԣ�H2O2�Ƕ�Ԫ���ᣬд��H2O2���뷽��ʽ________________________
��2������������ѡ��̪��ָʾ�����еζ���Ӧѡ��ͼ��________�ζ���(����)���ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�________��

A���ζ�����Һ��ı仯
B����ƿ����Һ��ɫ�ı仯
�ζ��յ���Һ��ɫ�ı仯��__________________________________________
��3��д���ҷ����ζ�ʱ�����ӷ���ʽ��___________________________________��
��4�����ҷ�����ȡ��Ʒ20g���100mL��Һ���ζ�����0.01000 mol��L��1 I2��Һ����������ʾ��
�ζ����� | ������Һ ���/mL | ����Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 20.00 | 1.02 | 21.03 |
2 | 20.00 | 2.00 | 25.00 |
3 | 20.00 | 0.60 | 20.59 |
�ζ������ϴ���ǵ�________��ʵ�飬����������Ŀ���ԭ����________��
A���ζ�����װҺǰδ�ñ���Һ��ϴ2��3��
B���ζ���ʼǰ�ζ��ܼ��첿�������ݣ��ڵζ��յ����ʱδ��������
C���ζ���ʼǰ�ζ��ܼ��첿��û�����ݣ��ڵζ��յ����ʱ���ּ��첿��������
D���ﵽ�ζ��յ�ʱ��������Һ��Һ����͵����
E���ζ������У���ƿҡ����̫���ң�������ЩҺ�ηɽ�����
��1 kg��Ʒ�к�SO2��������________g��
���𰸡� ǿ������ H2O2![]() HO2����H�� �� B ��ɫ��dz��ɫ�Ұ�����ڲ���ɫ HSO3����H2O��I2===3H����SO
HO2����H�� �� B ��ɫ��dz��ɫ�Ұ�����ڲ���ɫ HSO3����H2O��I2===3H����SO![]() ��2I�� �� SO2+I2+2H2O==4H++ SO
��2I�� �� SO2+I2+2H2O==4H++ SO![]() ��2I�� 2 A��B��D 3.2
��2I�� 2 A��B��D 3.2
����������1�������ڢٲ�������H2O2��ǿ��������H2O2�Ƕ�Ԫ���ᣬ���������������ƽ�⣬�Ե�һ������Ϊ����H2O2���뷽��ʽ�ɱ�ʾΪH2O2![]() HO2����H����
HO2����H����
��2�������ڢڲ�ѡ��̪��ָʾ�����еζ���Ӧѡ���ʽ�ζ��ܣ���ͼ���ҵζ��ܡ��ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��ѡB���ζ��յ���Һ��ɫ�ı仯����ɫ��Ϊdz��ɫ�Ұ�����ڲ���ɫ��
��3���ҷ����ζ�ʱ�����ӷ���ʽ��HSO3����H2O��I2===3H����SO![]() ��2I�� �� SO2+I2+2H2O==4H++ SO
��2I�� �� SO2+I2+2H2O==4H++ SO![]() ��2I����
��2I����
��4���ɱ������ݿ�֪����ͬ����Ĵ�����Һ����2�����ı���Һ�����Ϊ23.00mL�����Դ����������飬���ԣ��ζ������ϴ���ǵ�2��ʵ�飬����������Ŀ���ԭ�������ζ�����װҺǰδ�ñ���Һ��ϴ2��3�����ζ���ʼǰ�ζ��ܼ��첿�������ݣ��ڵζ��յ����ʱδ�����������ﵽ�ζ��յ�ʱ��������Һ��Һ����͵������ѡA��B��D�����ζ���ʼǰ�ζ��ܼ��첿��û�����ݣ��ڵζ��յ����ʱ���ּ��첿�����������������ı�Һ���ƫС�����ζ������У���ƿҡ����̫���ң�������ЩҺ�ηɽ���������ͬ���������ı�Һ���ƫС���ɵ�1��3�������20.00mL��Ʒ��Һƽ�����ı���Һ20.00mL���ɻ�ѧ����ʽ��֪��n(SO2)=n(I2)=20.00![]() 0.01000 mol��L��1 =2.00
0.01000 mol��L��1 =2.00![]() mol������20g��Ʒ�к�SO2������Ϊ2.00
mol������20g��Ʒ�к�SO2������Ϊ2.00![]() mol
mol![]() ����1 kg��Ʒ�к�SO2��������3.2g��
����1 kg��Ʒ�к�SO2��������3.2g��