��Ŀ����
����Ŀ������ijѧ����0.1000mol/L NaOH��Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
A��������ˮϴ�ɾ��ζ���
B���ô��ⶨ����Һ��ϴ��ʽ�ζ���
C������ʽ�ζ���ȡϡ����20. 00mL��ע����ƿ�У������̪
D����ȡ��ƿ�����ظ�����һ��
E�����ζ����Ƿ�©ˮ
F��ȡ�¼�ʽ�ζ����ñ���NaOH��Һ��ϴ����Һע���ʽ�ζ�����0���̶�����2��3 cm�����ٰѼ�ʽ�ζ��̶ܹ��ã�����Һ������0���̶Ȼ���0���̶�����
G������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶ȡ�
���������գ�
(1)��ȷ������˳����(�������ĸ��д)______________��
(2)�ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�_____________________________��
A���ζ�����Һ��ı仯 B����ƿ����Һ��ɫ�ı仯
�ζ��յ���Һ��ɫ�ı仯��__________________________________________��
(3)�ζ���������ʾ��
�ζ����� | ����Һ���/mL | ����Һ�����/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | |||
�� | 20 | 1.02 | 21.03 | |
�� | 20 | 2.00 | 25.00 | |
�� | 20 | 0.61 | 20.60 | |
�ζ������ϴ���ǵ�_______��ʵ�飬����������Ŀ���ԭ����_______��
A����ʽ�ζ�����װҺǰδ�ñ�NaOH��Һ��ϴ2��3��
B���ζ���ʼǰ��ʽ�ζ��ܼ��첿�������ݣ��ڵζ��յ����ʱδ��������
C���ζ���ʼǰ��ʽ�ζ��ܼ��첿��û�����ݣ��ڵζ��յ����ʱ���ּ��첿��������
D���ﵽ�ζ��յ�ʱ��������Һ��Һ����͵����
E���ζ������У���ƿҡ����̫���ң�������ЩҺ�ηɽ�����
(4)�������Ũ��Ϊ________mol/L��
(5)���ȷ��ȡ20. 00mL 0.1000mol/L NaOH��Һ����ƿ�У������ָ̪ʾ����Ȼ����δ֪Ũ�ȵ�����(װ����ʽ�ζ�����)�ζ����Ƿ�Ҳ�ɲⶨ����������ʵ���Ũ�ȣ�______________(����������������)��
���𰸡�EABCFGD B ��ɫ��dz��ɫ�Ұ�����ڲ���ɫ 2 A��B��D 0.1000 ��
��������
(1)�к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ���˳�������
(2)�ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����ɫ�仯���ζ��յ���Һ��ɫ�ı仯�ǣ���ɫ��dz��ɫ�Ұ�����ڲ���ɫ��
(3)��2��ʵ�����ϴ���������±�Һ���ƫ���Դ˷����жϣ�
(4)����2��Ч��ȡ1��3������м��㣬���ñ�Һ��ƽ�����Ϊ![]() mL=20.00mL���ɹ�ϵʽHCl��NaOH������ã�
mL=20.00mL���ɹ�ϵʽHCl��NaOH������ã�
(5)ȷ��ȡ20.00mL 0.1000mol/L NaOH��Һ����ƿ�У������ָ̪ʾ����Ȼ����δ֪Ũ�ȵ����ᣨװ����ʽ�ζ����У��ζ���ͨ����������������HCl��NaOH������������Ũ�ȡ�
(1)�к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ���˳�����������ȷ��˳��ΪEABCFGD���ʴ�Ϊ��EABCFGD��
(2)�ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����ɫ�仯���ζ��յ���Һ��ɫ�ı仯�ǣ���ɫ��dz��ɫ�Ұ�����ڲ���ɫ���ʴ�Ϊ��B����ɫ��dz��ɫ�Ұ�����ڲ���ɫ��
(3)��2��ʵ�����ϴ���������±�Һ���ƫ��
A. ��ʽ�ζ�����װҺǰδ�ñ�NaOH��Һ��ϴ2��3�Σ�����Ũ�ȱ�С��������Һ���ƫ��A��ȷ��
B. �ζ���ʼǰ��ʽ�ζ��ܼ��첿�������ݣ��ڵζ��յ����ʱδ�������ݣ����¶���ƫ��B��ȷ��
C. �ζ���ʼǰ��ʽ�ζ��ܼ��첿��û�����ݣ��ڵζ��յ����ʱ���ּ��첿�������ݣ����¶���ƫС����C����
D. �ﵽ�ζ��յ�ʱ��������Һ��Һ����͵���������¶���ƫ��D��ȷ��
E. �ζ������У���ƿҡ����̫���ң�������ЩҺ�ηɽ��������������ü�Һ���٣���E���ʴ�Ϊ��2��ABD��
(4)����2��Ч��ȡ1��3������м��㣬���ñ�Һ��ƽ�����Ϊ![]() mL=20.00mL���ɹ�ϵʽHCl��NaOH��֪n��HCl��=n��NaOH��=0.1000mol/L��0.02000L=0.002000mol��c��HCl��=
mL=20.00mL���ɹ�ϵʽHCl��NaOH��֪n��HCl��=n��NaOH��=0.1000mol/L��0.02000L=0.002000mol��c��HCl��= ![]() =0.1000mol/L���ʴ�Ϊ��0.1000��
=0.1000mol/L���ʴ�Ϊ��0.1000��
(5)ȷ��ȡ20.00mL 0.1000mol/L NaOH��Һ����ƿ�У������ָ̪ʾ����Ȼ����δ֪Ũ�ȵ����ᣨװ����ʽ�ζ����У��ζ���ͨ����������������HCl��NaOH������������Ũ�ȣ��ʴ�Ϊ���ǡ�

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�����Ŀ��Ӧ������к͵ζ�ԭ���ⶨij���۰״ĺ�����
I.ʵ�鲽��
(I)��ȡ10.00mLʳ�ð״ף�ϡ�͵�100mL���õ�����״���Һ��ȡ����״���Һ20.00mL����ƿ�У������еμӼ���________��ָʾ����
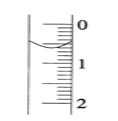
(2)��ʽ�ζ���ʢװ0.1000mol/LNaOH��Һ��ʼʱ��Һ��λ����ͼ��ʾ����ʱ�Ķ���_____mL��
(3)�ζ������У�����_________________ʱ��Ϊ�ζ��յ㣬��¼NaOH��Һ�����ն������ظ��ζ�4�Ρ�
��.ʵ���¼
ʵ����� | ����״���Һ���/mL | 0.1000mol/LNaOH��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 20.00 | 0.10 | l5.10 |
2 | 20.00 | 0.00 | 14.95 |
3 | 20.00 | 0.15 | 15.20 |
4 | 20.00 | 1.10 | 17.10 |
��.���ݴ���������
(4)��ʵ���������ݣ��������c(���۰״�)=__________mol/L��
(5)�ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����________(��ѡ����ĸ)��
a.��ʽ�ζ�����װҺǰδ�ñ�NaOH��Һ��ϴ
b.��ʽ�ζ��ܵļ����ڵζ�ǰ�����أ��ζ���������ʧ
c.��ƿ�м������״���Һ���ټ�����ˮ
d.��ƿ�ڵζ�ʱ����ҡ����������Һ�彦��


