��Ŀ����
����Ŀ��������þ������ҩ��ҵ��������Ҫ����ɫ��ȼ����
I������θ�����ҩ��Stmoache����Ч�ɷ�ΪMg(OH)2��
��1����ҩ������θ�ᣨ��Ҫ�ɷ�Ϊ���ᣩ����֢ʱ��Ӧ�����ӷ���ʽ��___________��
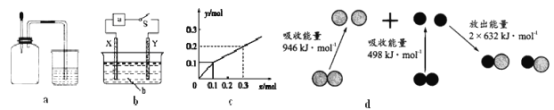
����֪��Mg (s)+2H2O(g)=Mg(OH)2(s)��H2��g�� ��H1=��441kJ��mol��1
H2O(g)=H2(g)��![]() O2(g) ��H2=+242kJ��mol��1
O2(g) ��H2=+242kJ��mol��1
Mg(s)��![]() O2(g)=MgO(s) ��H3=��602kJ��mol��1
O2(g)=MgO(s) ��H3=��602kJ��mol��1
��2��������þ�ֽ���Ȼ�ѧ����ʽ��___________��
��3��������þ������Ϊ��ȼ����ԭ��_________����дһ�����ɣ�
��ij��������ˮ���Ȼ�þ�ʹ�ʯ����ȡ��������þ�������������������ʣ�ͨ���������̽����ᴿ���ƣ������ȼ��������þ��
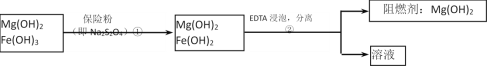
��4���������������������__________��
��5��������еķ�Ӧ���£�6Fe(OH)3 ��S2O42����2OH�� ��6Fe(OH)2 ��2SO42����4H2O��ÿ����0.1mol���շۣ�Na2S2O4��ʱ��ת�Ƶ��ӵ���Ŀ��__________mol��
��6����֪EDTAֻ������Һ�е�Fe2+��Ӧ����������ˮ�����ʣ�����Mg(OH)2��Ӧ����ȻFe(OH)2������ˮ���������������EDTA�ļ��룬�����ܹ���Fe(OH)2��ȥ����ô��ȸߵ�Mg(OH)2����ӳ����ܽ�ƽ��ĽǶȼ��Խ��͡�
_______________________________________��
���𰸡�Mg(OH)2+2H+=Mg2++2H2O Mg(OH)2(s)=MgO(s)+H2O(g) ��H=+81kJ��mol-1 ������þ�ֽ�Ҫ���մ������ȣ�����������Ҳ���ԣ� ���� 0.6 Fe(OH)2����Һ�д�������ƽ�⣺Fe(OH)2 ![]() Fe2++2OH-�������ϵ���EDTAʱ��EDTA�����Fe2+����ʹƽ�⳯���ƶ����ܽ�
Fe2++2OH-�������ϵ���EDTAʱ��EDTA�����Fe2+����ʹƽ�⳯���ƶ����ܽ�
��������
��1��������þ�ܺ����ᷢ���кͷ�Ӧ�����Ȼ�þ��ˮ��
��2�����ݸ�˹���������㷴Ӧ���ʱ䣻
��3��������þ�ֽ�����մ������ȣ�
��4��ʵ�ֹ����Һ��ķ�����ù��˵ķ�����
��5������������ԭ��Ӧ�е��ӵ�ת������ͻ��ϼ۵���������Ĺ�ϵ���ش�
��6�����ݻ�ѧƽ���ƶ�ԭ���������������Ũ����ʹ��ѧƽ����������Ӧ�����ƶ�֪ʶ���ش�
��1��������þ�ܺ����ᷢ���кͷ�Ӧ�������Ȼ�þ��ˮ��ʵ���ǣ�Mg��OH��2+2H+=Mg2++2H2O��
�ʴ�Ϊ��Mg��OH��2+2H+=Mg2++2H2O��
��2����Mg ��s��+2H2O��g��=Mg��OH��2��s��+H2��g�� ��H1=-441kJmol-1
��H2O��g��=H2��g��+![]() O2��g�� ��H2=+242kJmol-1
O2��g�� ��H2=+242kJmol-1
��Mg��s��+![]() O2��g��=MgO��s�� ��H3=-602kJmol-1
O2��g��=MgO��s�� ��H3=-602kJmol-1
���ݸ�˹���ɣ���ӦMg��OH��2��s��=MgO��s��+H2O��g�������Կ����Ǣ�+��-�٣����Ը÷�Ӧ�ġ�H=![]() =+81kJmol-1��
=+81kJmol-1��
�ʴ�Ϊ��Mg��OH��2��s��=MgO��s��+H2O��g�� ��H=+81kJmol-1��
��3��������þ�ֽ�����մ������ȣ�����������þ������Ϊ��ȼ����
�ʴ�Ϊ��������þ�ֽ�����մ������ȣ�
��4��EDTAֻ������Һ�е�Fe2+��Ӧ����������ˮ�����ʣ�����Mg��0H��2��Ӧ��ʵ��������þ����Һ����ķ����ǹ��ˣ�
�ʴ�Ϊ�����ˣ�
��5��������ԭ��Ӧ6Fe��OH��3+S2O42-+2OH-=6Fe��OH��2+2SO42-+4H2O�У����ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ�����=6����ÿ����1mol���շۣ�Na2S2O4��ʱ��ת�Ƶ���6mol����������0.1mol���շۣ�Na2S2O4��ʱ��ת�Ƶ���0.6mol��
�ʴ�Ϊ��0.6��
��6������EDTA�ļ��룬EDTA�����Fe2+�������������Ũ����ʹ��ѧƽ����������Ӧ�����ƶ���
�ʴ�Ϊ��Fe(OH)2����Һ�д�������ƽ�⣺Fe(OH)2 ![]() Fe2++2OH-�������ϵ���EDTAʱ��EDTA�����Fe2+����ʹƽ�⳯���ƶ����ܽ⡣
Fe2++2OH-�������ϵ���EDTAʱ��EDTA�����Fe2+����ʹƽ�⳯���ƶ����ܽ⡣

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д�