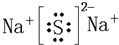��Ŀ����
�������ж�����Ԫ�����ʵ����ݣ�
��ش��������⣺
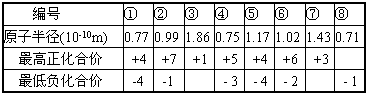
(1)���Ϊ�ݵ�Ԫ��ԭ�ӽṹʾ��ͼΪ________�������ڱ��е�λ����____________��˵�����������һ����;________________��
(2)����Ԫ���γɵĵ���Ӳ������Ԫ�ر����___________��
(3)����Ԫ������ۺ�����������ǿ��Ԫ����________��дԪ�ط��ţ���
(4)�ں͢�����Ԫ���γɵ���̬�⻯���У����ȶ�����_______________��д��ѧʽ����
(5)�ۺ͢�����Ԫ������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪ_______________��
(1)���Ϊ�ݵ�Ԫ��ԭ�ӽṹʾ��ͼΪ________�������ڱ��е�λ����____________��˵�����������һ����;________________��
(2)����Ԫ���γɵĵ���Ӳ������Ԫ�ر����___________��
(3)����Ԫ������ۺ�����������ǿ��Ԫ����________��дԪ�ط��ţ���
(4)�ں͢�����Ԫ���γɵ���̬�⻯���У����ȶ�����_______________��д��ѧʽ����
(5)�ۺ͢�����Ԫ������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪ_______________��
(1)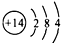 ���������ڵڢ�A�壻���ά�����ͻ���ϡ�����ȣ�
���������ڵڢ�A�壻���ά�����ͻ���ϡ�����ȣ�
(2)��
(3)Cl
(4)HF
(5)Al(OH)3+ NaOH=NaAlO2+2H2O
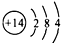 ���������ڵڢ�A�壻���ά�����ͻ���ϡ�����ȣ�
���������ڵڢ�A�壻���ά�����ͻ���ϡ�����ȣ� (2)��
(3)Cl
(4)HF
(5)Al(OH)3+ NaOH=NaAlO2+2H2O

��ϰ��ϵ�д�
�����Ŀ