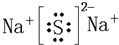��Ŀ����
�������ж�����Ԫ�����ʵ����ݣ�
�Իش��������⣺
��1��Ԫ�آ������ڱ���λ���� �� Ԫ�آ���Ԫ�آ���Ƚϣ���̬�⻯����ȶ����� ���ѧʽ����
��2��Ԫ�آ���Ԫ�آް���ԭ�Ӹ�����Ϊ1��1�γɵĻ�������ˮ��Ӧ�Ļ�ѧ����ʽ ��
��3��Ԫ�آ��γɵĵ��ʼ��뵽Ԫ�آڵ��⻯���ˮ��Һ�У���Ӧ��������ǿ������ӷ���ʽ ��
��4��Ԫ�آٵ��ʺ�Ԫ�آ���ں�ˮ�п����γ�ԭ��أ�д��������Ӧ ��
| Ԫ�ر�� Ԫ������ |
�� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶 ��10-10m�� |
0.74 | 1.02 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 1.43 |
| �����ͻ��ϼ� | +6 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -2 | -3 | -1 | -3 |
��1��Ԫ�آ������ڱ���λ����
��2��Ԫ�آ���Ԫ�آް���ԭ�Ӹ�����Ϊ1��1�γɵĻ�������ˮ��Ӧ�Ļ�ѧ����ʽ
��3��Ԫ�آ��γɵĵ��ʼ��뵽Ԫ�آڵ��⻯���ˮ��Һ�У���Ӧ��������ǿ������ӷ���ʽ
��4��Ԫ�آٵ��ʺ�Ԫ�آ���ں�ˮ�п����γ�ԭ��أ�д��������Ӧ
������������Ԫ�أ��٢ڶ���-2�ۣ����ڢ�A�壬����+6�ۣ����ΪO����ΪS���ۢ��������+1�����ڢ�A�壬�Ң�ԭ�Ӱ뾶�ϴ۵�ԭ�Ӱ뾶��������Ԫ������С�����ΪLi����ΪNa���ܢ߶���+5��-3�ۣ����ڢ�A�壬�ܵ�ԭ�Ӱ뾶�ϴ����ΪP����ΪN������+7��-1�ۣ����ΪClԪ�أ������������+3�����ڢ�A�壬ԭ�Ӱ뾶������Ԫ�أ����ΪAlԪ�أ��ݴ˽��
����⣺������Ԫ�أ��٢ڶ���-2�ۣ����ڢ�A�壬����+6�ۣ����ΪO����ΪS���ۢ��������+1�����ڢ�A�壬�Ң�ԭ�Ӱ뾶�ϴ۵�ԭ�Ӱ뾶��������Ԫ������С�����ΪLi����ΪNa���ܢ߶���+5��-3�ۣ����ڢ�A�壬�ܵ�ԭ�Ӱ뾶�ϴ����ΪP����ΪN������+7��-1�ۣ����ΪClԪ�أ������������+3�����ڢ�A�壬ԭ�Ӱ뾶������Ԫ�أ����ΪAlԪ�أ�
��1��Ԫ�آ�ΪLi�������ڱ���λ���ǵڶ����ڢ�A�壻 Ԫ�آ�ΪP��Ԫ�آ�ΪN���ǽ�����N��P������̬�⻯����ȶ�����NH3��
�ʴ�Ϊ���ڶ����ڢ�A�壻NH3��
��2��Ԫ�آ���Ԫ�آް���ԭ�Ӹ�����Ϊ1��1�γɵĻ�����ΪNa2O2����ˮ��Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
��3��Ԫ�آ��γɵĵ���ΪCl2��Ԫ�آڵ��⻯��ΪH2S��������H2Sˮ��Һ��Ӧ��������ǿ�ᣬӦ�������������ᣬ��Ӧ�����ӷ���ʽΪ��4Cl2+H2S+4H2O=10H++8Cl-+SO42-���ʴ�Ϊ��4Cl2+H2S+4H2O=10H++8Cl-+SO42-��
��4�������͵���Al�ں�ˮ���γ�ԭ��أ����ƽ�����������ʴ������������ԭ��Ӧ��������������õ��������������������缫��ӦʽΪ��O2+4e-+2H2O=4OH-��
�ʴ�Ϊ��O2+4e-+2H2O=4OH-��
��1��Ԫ�آ�ΪLi�������ڱ���λ���ǵڶ����ڢ�A�壻 Ԫ�آ�ΪP��Ԫ�آ�ΪN���ǽ�����N��P������̬�⻯����ȶ�����NH3��
�ʴ�Ϊ���ڶ����ڢ�A�壻NH3��
��2��Ԫ�آ���Ԫ�آް���ԭ�Ӹ�����Ϊ1��1�γɵĻ�����ΪNa2O2����ˮ��Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
��3��Ԫ�آ��γɵĵ���ΪCl2��Ԫ�آڵ��⻯��ΪH2S��������H2Sˮ��Һ��Ӧ��������ǿ�ᣬӦ�������������ᣬ��Ӧ�����ӷ���ʽΪ��4Cl2+H2S+4H2O=10H++8Cl-+SO42-���ʴ�Ϊ��4Cl2+H2S+4H2O=10H++8Cl-+SO42-��
��4�������͵���Al�ں�ˮ���γ�ԭ��أ����ƽ�����������ʴ������������ԭ��Ӧ��������������õ��������������������缫��ӦʽΪ��O2+4e-+2H2O=4OH-��
�ʴ�Ϊ��O2+4e-+2H2O=4OH-��
���������⿼��ṹ����λ�ù�ϵ���ã��Ѷ��еȣ����ݻ��ϼ���ԭ�Ӱ뾶�ƶ�Ԫ���ǽ���ؼ���ע��Ԫ�������ɵ��������գ�

��ϰ��ϵ�д�
 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ