��Ŀ����
����Ŀ��I.����480 mL 0.2mol/L NaOH��Һ���������£�
(1)��______gNaOH��
(2)���ƹ����У��������������õ�����__________(����ţ�����ȱ�ٵIJ���������______________��
A.������ƽ B.250mL����ƿ C.������ D.��ͷ�ι�
(3)ʵ������������õ��������������÷ֱ��ǣ�______________��_________��
(4)���ݲ�����������ˮע����ѡ�������У�ֱ������Һ��ӽ���̶���____________��ʱ������____________�μ�����ˮ����Һ��__________������̶���_______����ƿ���ӸǺã�����____________ҡ�ȡ�
(5)���в�������ȷ˳����(����ĸ��ʾ)B��_____��________��______��__________��_____��G��
A.ת�� B.���� C.���� D.ϴ�� E.��ȴ F.�ܽ� G.ҡ��
II.��ͬѧ��18.4mol/lŨ��������100ml 3.6mol/L��ϡ���ᡣ
��100 ml ��Ͳ��ȡ20 ml Ũ���ᣬ��������С�ĵؼ�������ˮ��������ȣ�����ȴ�����º��ټ���ˮ��100 ml �̶��ߣ��ٽ�����ȡ�
ָ�����д���֮��____________________________��
III.������һ�����ʵ���Ũ����Һʱ�����в������ֵĺ����(���ƫ����.��ƫ����.����Ӱ����)��
(1)��������������Һʱ����ȡ���������������ƹ��塣_____________��
(2)��������������Һʱ������ƿ��������ˮ��______________��
(3)������ҺҺ�泬���̶��ߣ���������������ˮ��ʹҺ�潵���̶�____________��
(4)ת��ʱ��������Һ����ƿ�⣻____________��
(5)����ʱ��������ƿ�̶��ߣ�____________��
���𰸡�4.0 B 500ml����ƿ���ձ� �����ܽ� ���� 1��2cm ��ͷ�ι� ��Һ�����͵� ���� ��תҡ�� F E A D C ����Ͳ��ϡ�ͣ�����ˮ�ӵ�Ũ������ ƫ�� ��Ӱ�� ƫ�� ƫ�� ƫ��
��������
I.(1)ѡ��ʹ��500mL����ƿ������m=cVM�������ʵ�������
(2)�����������ʵ���Ũ�ȵ���Һ�IJ���ȷ��ʹ�õ�������ȱ�ٵ����������ƣ�
(3)����������Һ�IJ���ȷ�������������ã�
(4)����������Һ�Ķ��ݲ���������д��
(5)�������ʵ���Ũ�ȵ���Һ�IJ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�ȡ�
II.�������������ü�Ũ��������ˮ�ų�����������
III.�������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
��.�����ڻ�ѧʵ������û��480 mL������ƿ������ֻ����500 mL������ƿ��m=nV��n=cV������V=500 mL=0.5L������ҪNaOH������m(NaOH)= 0.2mol/L ��0.5L��40g/mol=4.0g��
�������ƹ����У�������ƽ����������NaOH����������Ҫ��500 mL������ƿ�����ձ����ܽ����ҩƷʱҪ�ò��������裬ת����ҺʱҪʹ�ò�������������ͷ�ι��ڶ���ʱ�õ����ɼ�����ʹ��250mL����ƿ���ʺ���ѡ����B��ȱ�ٵ���500 mL����ƿ���ձ���
�ǵ�һ���ò����������ܽ�ʱ�ã������ǽ��裬���ٹ��������ܽ⣻�ڶ���ʹ�ò�������������������
���ڶ���ʱ����Һ��ӽ��̶���1��2cmʱӦ�ý�ͷ�ιܵμӣ���Һ�尼Һ�����ʹ��̶�������ʱֹͣ�μӣ�Ȼ�����·�����תҡ�ȣ��͵õ���500mL0.2mol/L��NaOH��Һ��
��������Һ��һ�㲽��Ϊ���������ܽ�����ȴ��������ת����ϴ����������ҡ�ȣ��ʺ���˳���ǣ�B��F��E��A��D�� C��G��
II.��ͲΪ������������������ϡ��Ũ��Һ��Ũ����ϡ�Ͳ����������ȣ�ϡ��ʱӦ��Ũ���Ỻ��ע��ˮ�У����Դ���֮�����ڣ�����Ͳ��ϡ�ͣ�����ˮ�ӵ�Ũ�����У�
III.(1)��������������Һʱ����ȡ���������������ƹ��壬���³�ȡ���������������ʵ���ƫС����ҺŨ��ƫ�ͣ�
(2)��������������Һʱ������ƿ��������ˮ�����������ʵ�������Һ�����������Ӱ�죬��ҺŨ����Ӱ�죻
(3)������ҺҺ�泬���̶��ߣ���������������ˮ��ʹҺ�潵���̶ȣ����²���������ģ��������ʵ���ƫС�������Ƶ���ҺŨ��ƫ�ͣ�
(4)ת��ʱ��������Һ����ƿ�⣬���²���������ģ��������ʵ���ƫС����ҺŨ��ƫ�ͣ�
(5)����ʱ��������ƿ�̶��ߣ�������Һ���ƫС��ʹ��ҺŨ��ƫ�ߡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���о�ú�ĺ������ü�CO2���ۺ�Ӧ��������Ҫ�����塣��ش���������:
I.ú������
��֪ú�����������漰�Ļ�����ѧ��Ӧ�У�
C��s��+H20��g��![]() CO��g��+H2��g�� ��H=+131kJ/mol
CO��g��+H2��g�� ��H=+131kJ/mol
��CO��g��+3H2��g��![]() CH4��g��+H2O��g�� ��H=akJ/mol
CH4��g��+H2O��g�� ��H=akJ/mol
�������Ϸ�Ӧ������ػ�ѧ�������������±���
��ѧ�� |
| H-H | H��C | H-O |
E/��kJ/mol�� | 1072 | 436 | 414 | 465 |
��1��úֱ�Ӽ��黯��ӦC��s��+2H2��g��![]() CH4��g���ġ�HΪ_______kJ/mol���÷�Ӧ��______����{�¡����¡������Է����С�
CH4��g���ġ�HΪ_______kJ/mol���÷�Ӧ��______����{�¡����¡������Է����С�
��.�ϳɵ�̼ϩ��
�����Ϊ1 L���ܱ������У�����1molCO2��2.5molH2��������Ӧ��
2C02��g��+6H2��g��![]() C2H4��g��+4H20��g�� ��H=-128kJ/mol������¶ȶԴ�����Ч�ʺ� C02ƽ��ת���ʵ�Ӱ����ͼ��ʾ��
C2H4��g��+4H20��g�� ��H=-128kJ/mol������¶ȶԴ�����Ч�ʺ� C02ƽ��ת���ʵ�Ӱ����ͼ��ʾ��
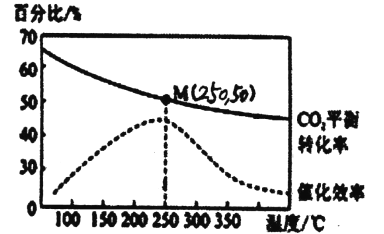
��2��ͼ�е���ʱ�������¶����ߴ����Ĵ�Ч����ߣ���C02��ƽ��ת����ȴ�������ͣ���ԭ����__________��
��3��250��ʱ���÷�Ӧ��ƽ�ⳣ��KֵΪ__________��
��.�ϳɼ״�
�ں���2 L�ݻ�������ܱ������У�����lmolC02��3molH2��������Ӧ��C02��g��+3H2��g��![]() CH30H��g��+H20��g������ò�ͬʱ�̷�Ӧǰ��������ѹǿ�仯��p��/pǰ�����±���
CH30H��g��+H20��g������ò�ͬʱ�̷�Ӧǰ��������ѹǿ�仯��p��/pǰ�����±���
ʱ��/h | 1 | 2 | 3 | 4 | 5 | 6 |
p��/pǰ | 0.90 | 0.85 | 0.82 | 0.81 | 0.80 | 0.80 |
��4����Ӧǰ1Сʱ�ڵ�ƽ����Ӧ����V��H2��Ϊ_________mol/��L��h�������¶���C02��ƽ��ת����Ϊ_________��
IV.�����ת�����Ҵ�
��5��������Աͨ������ʵ�鷢�֣�C02����������ˮ��Һ�е�������Ҵ����������Ҵ��ķ�Ӧ������_________���������������������õ缫�ķ�ӦʽΪ_________��