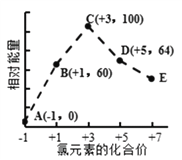
��Ŀ����
����Ŀ����1����֪��P4(s)+6Cl2(g)![]() 4PCl3(g)����H=akJ��mol-1��P4(s)+10Cl2(g)
4PCl3(g)����H=akJ��mol-1��P4(s)+10Cl2(g)![]() 4PCl5(g) ��H=bkJ��mol-1���ƻ�PCl5��1mol P��Cl����������ΪckJ��mol-1���ƻ�PCl3��1molP��Cl����������Ϊ1.2ckJ��mol-1�����ƻ�Cl2��1molCl��Cl�����������Ϊ___________________��
4PCl5(g) ��H=bkJ��mol-1���ƻ�PCl5��1mol P��Cl����������ΪckJ��mol-1���ƻ�PCl3��1molP��Cl����������Ϊ1.2ckJ��mol-1�����ƻ�Cl2��1molCl��Cl�����������Ϊ___________________��
��2����ҵ�ϳɰ�ʱ���ϳ�����ÿ����1molNH3�ų�46kJ���������䷴Ӧ���̵������仯��ͼ��bֵΪ________kJ�����������aֵ____(��������������С������������,��ͬ)��ѹ�����������bֵ___��

��3�����϶����������ʹ��Һ̬������������Һ̬ƫ������(C2H8N2)���ƽ�����N2O4��ƫ������ȼ�ղ���ֻ��CO2(g)��H2O(g)��N2(g)�����ų������ȣ���֪10.0 gҺ̬ƫ��������Һ̬������������ȫȼ�տɷų�425 kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ_____________________________��
���𰸡�![]() 92 ��С ���� C2H8N2(l)��2N2O4(l)===2CO2(g)��4H2O(g)��3N2(g)����H����2 550.0 kJ��mol��1
92 ��С ���� C2H8N2(l)��2N2O4(l)===2CO2(g)��4H2O(g)��3N2(g)����H����2 550.0 kJ��mol��1
��������
(1)��P4(s)+6Cl2(g)�T4PCl3(g)��H=a kJmol-1����P4(s)+10Cl2(g)�T4PCl5(g)��H=b kJmol-1�����ø�˹���� ![]() �ã�Cl2(g)+PCl3(g)=PCl5(g)��H=
�ã�Cl2(g)+PCl3(g)=PCl5(g)��H= ![]() kJmol-1���ٽ���ʱ�=��Ӧ�����֮��-���������֮�ͷ������
kJmol-1���ٽ���ʱ�=��Ӧ�����֮��-���������֮�ͷ������
(2)��������ͼ��֪bֵΪ1mol������3mol������ȫ��Ӧ����2mol NH3�ų���������������������ͷ�Ӧ�Ļ�ܣ�ѹ�����������ƽ�ⷢ���ƶ�������Ӧ�Ȳ��䣬�ݴ˽����
(3)�������ʵ��������������ȣ��Լ��Ȼ�ѧ����ʽ����д�����������
(1)��P4(s)+6Cl2(g)�T4PCl3(g)��H=a kJmol-1����P4(s)+10Cl2(g)�T4PCl5(g)��H=b kJmol-1�����ø�˹���� ![]() �ã�Cl2(g)+PCl3(g)=PCl5(g)��H=
�ã�Cl2(g)+PCl3(g)=PCl5(g)��H= ![]() kJmol-1���ʱ�=��Ӧ�����֮��-���������֮�ͣ��ɵã�E(Cl-Cl)+3��1.2c-5c=
kJmol-1���ʱ�=��Ӧ�����֮��-���������֮�ͣ��ɵã�E(Cl-Cl)+3��1.2c-5c= ![]() kJmol-1�����E(Cl-Cl)=
kJmol-1�����E(Cl-Cl)= ![]() kJmol-1���ʴ�Ϊ��
kJmol-1���ʴ�Ϊ��![]() ��
��
(2)��������ͼ��֪bֵΪ1mol������3mol������ȫ��Ӧ����2mol NH3�ų�����������ÿ����1mol NH3���ų�46kJ������������b=46��2=92��������������ͷ�Ӧ�Ļ�ܣ���a��С�������֮��䣬��Ӧ��b���䣻ѹ�����������ƽ��ᷢ���ƶ�������Ӧ�Ȳ��䣬��bֵ���䣬�ʴ�Ϊ��92����С�����䣻
(3)10.0 gҺ̬ƫ��������Һ̬������������ȫȼ�տɷų�425 kJ��������1molҺ̬ƫ��������������Һ̬��������������ȫ��Ӧ����N2(g)��CO2(g)��H2O(g)���ų�������Ϊ![]() ��425 kJ =2250kJ�����Ȼ�ѧ����ʽΪ��C2H8N2(l)+2N2O4(l)=3N2(g)+2CO2(g)+4H2O(g) ��H=-2250kJ/mol���ʴ�Ϊ��C2H8N2(l)+2N2O4(l)=3N2(g)+2CO2(g)+4H2O(g) ��H=-2250kJ/mol��
��425 kJ =2250kJ�����Ȼ�ѧ����ʽΪ��C2H8N2(l)+2N2O4(l)=3N2(g)+2CO2(g)+4H2O(g) ��H=-2250kJ/mol���ʴ�Ϊ��C2H8N2(l)+2N2O4(l)=3N2(g)+2CO2(g)+4H2O(g) ��H=-2250kJ/mol��

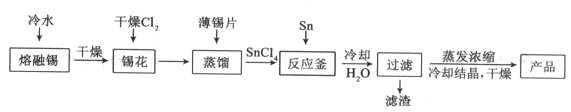
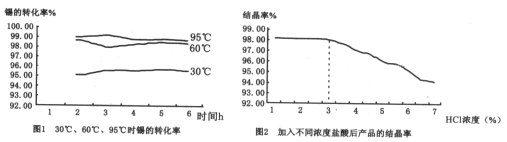
����Ŀ���������Ͼ��и�Ӳ�ȡ���ǿ�ȡ������ԡ����ߵ���ĥ�Լ��ͻ�ѧ��ʴ�Ե��������ﻯ���ܡ����Ӣʯ(��Ҫ�ɷ�Ϊ ZrSiO4����������Al2O3��SiO2��Fe2O3������)Ϊԭ��ͨ�����۷��Ʊ������(ZrO2)���������£�

25��ʱ���й�������ˮ��Һ�г���ʱ��pH���ݣ�
Fe(OH)3 | Zr(OH)4 | Al(OH)3 | |
��ʼ����ʱpH | 1.9 | 2.2 | 3.4 |
������ȫʱpH | 3.2 | 3.2 | 4.7 |
��ش��������⣺
(1)������ּ�����ѧ��Ӧ���ʵĴ�ʩ��___________��
(2)����I��������___________����2�ijɷ�Ϊ___________��
(3)�Ӣʯ��������������ת��ΪNa2ZrO3��д���÷�Ӧ�Ļ�ѧ����ʽ��___________��
(4)������pH��ʱ�����ʵ�pH��Χ��___________��Ϊ�˵õ�����ZrO2��Zr(OH)4��Ҫϴ�ӣ�����Zr(OH)4�Ƿ�ϴ�Ӹɾ��ķ�����___________��
(5)д�����������������̵Ļ�ѧ����ʽ___________������ZrO2�����ʣ��Ʋ���������;___________��
����Ŀ����1L�����ܱ������г���X(g)��Y(g)��������ӦX(g)+Y(g) ![]() M(g)+N(g)������ʵ���������±�������˵������ȷ����
M(g)+N(g)������ʵ���������±�������˵������ȷ����
ʵ���� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | |
n(X) | n(Y) | n(M) | ||
�� | 700 | 0.10 | 0.10 | 0.09 |
�� | 800 | 0.20 | 0.20 | 0.10 |
�� | 900 | 0.10 | 0.15 | a |
A. ����ӦΪ���ȷ�Ӧ
B. ʵ����У���5minʱ���n(M)=0.05mol����0~5minʱ���ڣ���N��ʾ��ƽ����Ӧ����v��N��= 0.01mol/(Lmin)
C. ʵ����У��÷�Ӧ��ƽ�ⳣ��K=1.0
D. ʵ����У��ﵽƽ��ʱ��a����0.06
����Ŀ����1��25 ��ʱ���Ʊ������������漰���Ȼ�ѧ����ʽ��ƽ�ⳣ�������
�Ȼ�ѧ����ʽ | ƽ�ⳣ�� | |
�� | 2NO2(g)+NaCl(s) | K1 |
�� | 4NO2(g)+2NaCl(s) | K2 |
�� | 2NO(g)+Cl2(g) | K3 |
����¶��£���H3=_______________kJmol-1��K3=_____________����K1��K2��ʾ����
��2��25��ʱ�������Ϊ2L�ĺ����ܱ�������ͨ��0.08 mol NO��0.04 molCl2����������Ӧ�ۣ�����Ӧ��ʼ�����ʱ�¶���ͬ������ѹǿ����ʾ��Ӧ������ѹǿ(p)��ʱ��(t)�ı仯��ͼ��ʵ����ʾ����H3 ___���>����<����=����0��������������ͬ�����ı�ijһ�����������ѹǿ��ʱ��ı仯��ͼ��������ʾ����ı��������_____________����5 minʱ���ٳ���0.08 mol NO��0.04 molCl2�����������ƽ����Է���������_____________�����������С�����䡱����ͼ���Ǽס�����ͬѧ���������Ӧ�۵�ƽ�ⳣ���Ķ���ֵ��lgK�����¶ȵı仯��ϵͼ��������ȷ��������______����ס����ҡ�����aֵΪ__________��25 ��ʱ��÷�Ӧ����ijʱ�̣�NO(g)��Cl2(g)��NOCl(g)��Ũ�ȷֱ�Ϊ0.8��0.1��0.3�����ʱv��_________v�����>����������=����
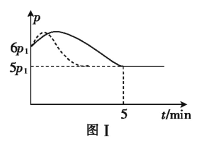

(3)��300 �桢8 MPa�£���CO2��H2�����ʵ���֮��1��3 ͨ��һ�ܱ������з���CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)�з�Ӧ���ﵽƽ��ʱ�����CO2��ƽ��ת����Ϊ50%����÷�Ӧ�����µ�ƽ�ⳣ��ΪKp��_____(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
CH3OH(g)��H2O(g)�з�Ӧ���ﵽƽ��ʱ�����CO2��ƽ��ת����Ϊ50%����÷�Ӧ�����µ�ƽ�ⳣ��ΪKp��_____(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��