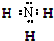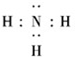��Ŀ����
��֪X��Y��Z��W�Ƕ�����Ԫ���е����ַǽ���Ԫ�أ����ǵ�ԭ��������������XԪ�ص�ԭ���γɵ����Ӿ���һ�����ӣ�Z��W��Ԫ�����ڱ��д������ڵ�λ�ã����ǵĵ����ڳ����¾�Ϊ��ɫ���壬Yԭ�ӵ��������������ڲ��������2����
(1)��д��Z��Ԫ�����ڱ��е�λ��________��
(2)��һ�������£���X������Z���ʷ�Ӧ����1 mol E�ų�������Ϊ46.2 kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ��__________________________________��E�ڴ������ڵ������£������ڻ�ԭ����β���е�________________���Լ��ٶԴ�������Ⱦ��
(3)����X��Z��W����Ԫ����ɵ�ij������һ����Ч���ʣ�������ʩ�û�ʹ�����ữ���йص����ӷ���ʽΪ___________________________________����X��Y��Z��W����Ԫ�ؿ������ʽ�Σ��û������ˮ��Һ������NaOH��Һ�ڼ��������·�Ӧ�����ӷ���ʽΪ________________________��
(4)��ҵ����E�������������Ĺܵ��Ƿ�©�����ɹ۲쵽�������̣�ͬʱ�е���Z���ɣ�д����ѧ����ʽ________________________________���÷�Ӧ�б�������E����뷴Ӧ��E������֮��________________________��
(1)�ڶ����ڣ��ڢ�A��
(2)N2(g)��3H2(g)  2NH3(g)����H����92.4 kJ��mol��1�� NO��NO2
2NH3(g)����H����92.4 kJ��mol��1�� NO��NO2
(3)NH4+��H2O NH3��H2O��H����NH4+��HCO3����2OH��
NH3��H2O��H����NH4+��HCO3����2OH�� NH3����CO32����2H2O
NH3����CO32����2H2O
(4)8NH3��3Cl2===6NH4Cl��N2��1��4
����������1������Ԫ�صĽṹ���й����ʿ�֪��X��Y��Z��W�ֱ���H��C��N��O����Ԫ�ص�ԭ��������7��λ�����ڱ��ĵڶ����ڵڢ�A�塣
��2�������͵�����Ӧ���ɵ��ǰ���������1mol����������46.2kJ�����Ը÷�Ӧ���Ȼ�ѧ����ʽ��N2(g)��3H2(g)  2NH3(g)����H����92.4 kJ��mol��1������β���е�NO��NO2���������������ɵ������Ӷ����ٶԴ�������Ⱦ��
2NH3(g)����H����92.4 kJ��mol��1������β���е�NO��NO2���������������ɵ������Ӷ����ٶԴ�������Ⱦ��
��3���û���������泥�NH4+ˮ�������ԣ�����ʽ��NH4+��H2O NH3��H2O��H��������Ԫ����ɵ���ʽ����̼����泥��������������Ʒ�Ӧ�ķ���ʽ��NH4+��HCO3����2OH��
NH3��H2O��H��������Ԫ����ɵ���ʽ����̼����泥��������������Ʒ�Ӧ�ķ���ʽ��NH4+��HCO3����2OH�� NH3����CO32����2H2O��
NH3����CO32����2H2O��
��4���д����İ���˵���а������ɣ���˷�Ӧ�ķ���ʽ��8NH3��3Cl2===6NH4Cl��N2�����ݷ���ʽ��֪��8mol�����μӷ�Ӧ������������2mol�����Ա�ֵ��1��4��

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
 ��֪X��Y��Z��W�Ƕ����������ַǽ���Ԫ�أ����ǵ�ԭ��������������XԪ��ԭ�Ӽ۵����Ų�ʽΪns1��YԪ���ǿ����к�������Ԫ�أ�Z ��Wͬ���壬����Wԭ�ӵĵ����Ų�ʽ�У�p�����ֻ��1��δ�ɶԵ��ӣ�
��֪X��Y��Z��W�Ƕ����������ַǽ���Ԫ�أ����ǵ�ԭ��������������XԪ��ԭ�Ӽ۵����Ų�ʽΪns1��YԪ���ǿ����к�������Ԫ�أ�Z ��Wͬ���壬����Wԭ�ӵĵ����Ų�ʽ�У�p�����ֻ��1��δ�ɶԵ��ӣ�