��Ŀ����
 ��ͼװ��ʵ�飬A��B���ձ��ֱ�ʢ��200g10%NaOH������CuSO4��Һ��ͨ��һ��ʱ���c������Cu�������ֲ��A������Һ����������4.5g��������ˮ����������
��ͼװ��ʵ�飬A��B���ձ��ֱ�ʢ��200g10%NaOH������CuSO4��Һ��ͨ��һ��ʱ���c������Cu�������ֲ��A������Һ����������4.5g��������ˮ������������ش��������⣺
��1����ԴP��Ϊ
��
��
������ֱ�д��b����c���Ϸ����ĵ缫��Ӧʽ��4OH--4e-=2H2O+O2��
4OH--4e-=2H2O+O2��
��Cu2++2e-=Cu
Cu2++2e-=Cu
��2��c������������ͭ������Ϊ
16
16
g��3����װ������Ǧ��������Դ����֪Ǧ���طŵ�ʱ�������·�Ӧ��
������Pb+SO42-=PbSO4+2e-
������PbO2+4H++SO42-+2e-=PbSO4+2H2O
������a���Ƶ�����0.050mol����ʱ��������ĵ�H2SO4�����ʵ���������
0.10
0.10
mol��������c������Cu������˵��cΪ���ص�������dΪ��������PΪ������QΪ������AΪ���NaOH��Һ��ʵ����Ϊ�缫ˮ��BΪ�������ͭ��Һ������������ͭ���缫��Ӧ��Cu2++2e-=Cu��������������������4OH--4e-=2H2O+O2������ϵ缫����ʽ���㣮
����⣺��1��c������Cu������˵��cΪ���ص�������dΪ��������PΪ������bΪ���NaOH��Һ������������ӦΪ4OH--4e-=2H2O+O2����cΪ�������ͭ��Һ��������������ӦΪCu2++2e-=Cu��
�ʴ�Ϊ������4OH--4e-=2H2O+O2����Cu2++2e-=Cu��
��2��A�ܷ�ӦʽΪ2H2O
2H2��+O2����A������Һ����������4.5g��ӦΪ����ˮ��������n��H2O��=
=0.25mol��ת�Ƶ���Ϊ0.5mol��c������������ͭ�����ʵ���Ϊ0.25mol������Ϊ0.25mol��64g/mol=16g��
�ʴ�Ϊ��16��
��3��a���Ƶ�����0.050mol����Ϊ������ת�Ƶ���0.10mol��Ǧ�����ܷ�ӦʽΪPb+PbO2+2H2SO4=2PbSO4+2H2O�����������ĵ�H2SO4�����ʵ���������0.10mol��
�ʴ�Ϊ��0.10��
�ʴ�Ϊ������4OH--4e-=2H2O+O2����Cu2++2e-=Cu��
��2��A�ܷ�ӦʽΪ2H2O
| ||
| 4.5g |
| 18g/mol |
�ʴ�Ϊ��16��
��3��a���Ƶ�����0.050mol����Ϊ������ת�Ƶ���0.10mol��Ǧ�����ܷ�ӦʽΪPb+PbO2+2H2SO4=2PbSO4+2H2O�����������ĵ�H2SO4�����ʵ���������0.10mol��
�ʴ�Ϊ��0.10��
����������Ϊ�绯ѧ֪ʶ���ۺ�Ӧ�ã�����ʱҪע����ݵ缫��Ӧ�����жϳ����ص��������������жϳ���Դ����������Ҫע����������Ϊ������·�����缫�ϵ�ʧ���ӵ���Ŀ��ȣ�����ʱҪ��ȷд���缫����ʽ��ȷ�ж����������ӵķŵ�˳��

��ϰ��ϵ�д�
 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�
�����Ŀ
 ��ͼװ��ʵ�飬A��B���ձ��ֱ�ʢ��200g10%KOH������CuSO4��Һ��ͨ��һ��ʱ���c������3.2g��
��ͼװ��ʵ�飬A��B���ձ��ֱ�ʢ��200g10%KOH������CuSO4��Һ��ͨ��һ��ʱ���c������3.2g��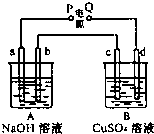 ����ͼװ��ʵ�飬A��B���ձ��ֱ�ʢ��200g10%NaOH������CuSO4��Һ��ͨ��һ��ʱ���c������Cu�������ֲ��A������Һ����������4.5g ��������ˮ����������a��b��cΪʯī�缫��dΪͭ�缫��
����ͼװ��ʵ�飬A��B���ձ��ֱ�ʢ��200g10%NaOH������CuSO4��Һ��ͨ��һ��ʱ���c������Cu�������ֲ��A������Һ����������4.5g ��������ˮ����������a��b��cΪʯī�缫��dΪͭ�缫��
