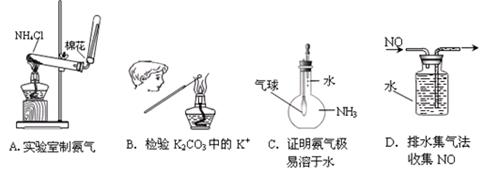
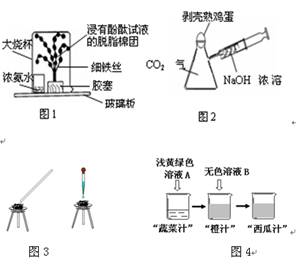
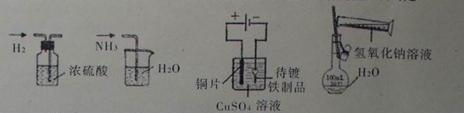
��Ŀ����
��12�֣�ij��ѧʵ��С����̵���ص�Ũ�����Լ���ǩ�IJ����������������Լ�ƿ���ܷ�ǩ���������ǻ��ɸ�����������������ǩ������������չ�о������������ǩ����Ϊ�����ѧ��(CP)��Ʒ��������, ��ѧʽ��H2SO4 ��Է���������98,����������98% �ܶȣ�1.98 g/cm3��
��ͬѧ��Ϊ��������һ�־���������������Һ��c(H��)����c(H��)��36.8 mol��L��1�������Һ����������Ϊ98%��
��ͬѧ��Ϊ����ʹ�о��ܵ���������ͬѧ�ķ���Ҳ���У��������������������ⶨ���������룺ȡһ������ĸ������������Ȼ�����Һ��Ӧ�����ˡ�ϴ�ӡ��������������������������
��ͬѧ�������к͵ζ������вⶨ���������£���ȷ��ȡһ����������ᣬ��������ˮϡ�ͣ�����ϡ�ͺ����Һ�е��뼸��ָʾ�������ü�ʽ�ζ�����ȡ��Ũ�ȵ�����������Һ�ζ���ֱ�����ֵζ��յ�Ϊֹ���ܼ�¼���ĵ�����������Һ�������
��ش��������⣺(1)��ͬѧ�Ʋ��ͬѧ�ķ��������е�������______________��1�֣�
(2)��ͬѧ�ķ����Ĺؼ����������㣺��ȷ��SO��ȫ��������ϴ�ӳ�����ȷ�������������ʡ���ʵ���У�ϴ�ӳ����IJ���������________________________________________��,
��Ƽ�ʵ�����SO�Ƿ���ȫ������_________________________________��2�֣�
(3)�ڱ�ͬѧ�ķ����в�����õ���ָʾ����________���ﵽ�ζ��յ�ı�־��________________________________________________________________________����2�֣�
(4)��ͬѧ�ķ����У����в����Բⶨ�����Ӱ����ȷ������________����2�֣�
���ڵζ�ǰδ��NaOH����Һ��ϴ�ζ���,�ڵζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���ζ��ܼ��첿����������,����ƿ������ˮϴ����û���ô���Һ��ϴ,�ܵζ�ǰ�����Ӷ������ζ��������Ӷ���,�ݵζ�ǰ�����Ӷ������ζ����Ӷ���
(5)��ͬѧ��ʵ���У��ֱ�ȡ�����ԭ���ᣬ��NaOH��Һ�ζ����Σ��յ�ʱ���õ���NaOH��Һ������±���ʾ��,
ͨ�����㣬ȷ�ϸ�Ũ���������������ǩ�Ƿ������________(�������������)�����ɣ����㲽�裩��______________________________________________________����5�֣�
��ͬѧ��Ϊ��������һ�־���������������Һ��c(H��)����c(H��)��36.8 mol��L��1�������Һ����������Ϊ98%��
��ͬѧ��Ϊ����ʹ�о��ܵ���������ͬѧ�ķ���Ҳ���У��������������������ⶨ���������룺ȡһ������ĸ������������Ȼ�����Һ��Ӧ�����ˡ�ϴ�ӡ��������������������������
��ͬѧ�������к͵ζ������вⶨ���������£���ȷ��ȡһ����������ᣬ��������ˮϡ�ͣ�����ϡ�ͺ����Һ�е��뼸��ָʾ�������ü�ʽ�ζ�����ȡ��Ũ�ȵ�����������Һ�ζ���ֱ�����ֵζ��յ�Ϊֹ���ܼ�¼���ĵ�����������Һ�������
��ش��������⣺(1)��ͬѧ�Ʋ��ͬѧ�ķ��������е�������______________��1�֣�
(2)��ͬѧ�ķ����Ĺؼ����������㣺��ȷ��SO��ȫ��������ϴ�ӳ�����ȷ�������������ʡ���ʵ���У�ϴ�ӳ����IJ���������________________________________________��,
��Ƽ�ʵ�����SO�Ƿ���ȫ������_________________________________��2�֣�
(3)�ڱ�ͬѧ�ķ����в�����õ���ָʾ����________���ﵽ�ζ��յ�ı�־��________________________________________________________________________����2�֣�
(4)��ͬѧ�ķ����У����в����Բⶨ�����Ӱ����ȷ������________����2�֣�
���ڵζ�ǰδ��NaOH����Һ��ϴ�ζ���,�ڵζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���ζ��ܼ��첿����������,����ƿ������ˮϴ����û���ô���Һ��ϴ,�ܵζ�ǰ�����Ӷ������ζ��������Ӷ���,�ݵζ�ǰ�����Ӷ������ζ����Ӷ���
(5)��ͬѧ��ʵ���У��ֱ�ȡ�����ԭ���ᣬ��NaOH��Һ�ζ����Σ��յ�ʱ���õ���NaOH��Һ������±���ʾ��,
| ʵ����� | ������� | NaOH��Һ��� | NaOH��ҺŨ�� |
| �� | 5.00 mL | 35.65 mL | 5.00 mol��L-1 |
| �� | 5.00 mL | 39.65 mL | 5.00 mol��L-1 |
| �� | 5.00 mL | 35.55 mL | 5.00mol��L-1 |
(1)Ũ���Ậˮ�٣�������Ҫ�Է�����ʽ����
(2)ͨ�����������������������ע������ˮ����û��������ˮ��������ע������ˮ���ظ����ֱ������ϴ��Ϊֹ�����ϲ���Һ�еμ��Ȼ���(�����ᱵ��)��Һ����������ɫ��������˵��SOû����ȫ����������������ɫ��������˵��SO����ȫ����
(3)��̪��Һ����ɫ��ɷۺ�ɫ���Ұ�����ڲ���ɫ,(4)�ڢ�,(5)���������ȥ���������(VNaOH��39.65 mL)���ɵã���35.60 mL�����ݱ�ǩ���ݼ����Ũ��������ʵ���Ũ��Ϊc(H2SO4)�� mol��L��1��19.8mol��L��1�������к͵ζ����Ũ�����Ũ��Ϊc(H2SO4)��mol��L��1��17.8mol��L��1<19.8mol��L��1
(2)ͨ�����������������������ע������ˮ����û��������ˮ��������ע������ˮ���ظ����ֱ������ϴ��Ϊֹ�����ϲ���Һ�еμ��Ȼ���(�����ᱵ��)��Һ����������ɫ��������˵��SOû����ȫ����������������ɫ��������˵��SO����ȫ����
(3)��̪��Һ����ɫ��ɷۺ�ɫ���Ұ�����ڲ���ɫ,(4)�ڢ�,(5)���������ȥ���������(VNaOH��39.65 mL)���ɵã���35.60 mL�����ݱ�ǩ���ݼ����Ũ��������ʵ���Ũ��Ϊc(H2SO4)�� mol��L��1��19.8mol��L��1�������к͵ζ����Ũ�����Ũ��Ϊc(H2SO4)��mol��L��1��17.8mol��L��1<19.8mol��L��1
���⿼��̽���Ļ������������Ũ��������ʣ����ζ��IJ����ͼ��㣬���ˡ�ϴ�ӵĻ���������(1)��ΪŨ���Ậˮ�٣��������ܵ����H+����Ҫ�Է�����ʽ���ڣ��ʲ��ܲⶨ��
(2)ͨ�����������������������ע������ˮ����û��������ˮ��������ע������ˮ���ظ����ֱ������ϴ��Ϊֹ�����ϲ���Һ�еμ��Ȼ���(�����ᱵ��)��Һ����������ɫ��������˵��SOû����ȫ����������������ɫ��������˵��SO����ȫ����
(3)ǿ��ǿ��ĵζ�����ʹ�÷�̪�����ȣ�
(4)����δ��ϴ������NaOHŨ�ȼ�С������������ⶨ���ƫ�ߣ��ڵζ�ǰ�������ݣ�������ռ�������ȷ�����ʲ���ȷ������Ϊ��ȷ������û��Ӱ�죬������������ӣ������ӵĽǶ��Ƿ�һ����ͬ������ȷ�����ݵζ�Ǯ���ӣ��ζ����ӣ������ƫС���ⶨŨ��ƫС���ʴ�Ϊ�ڢܣ�
(5)���������ȥ���������(VNaOH��39.65 mL)���ɵã���35.60 mL�����ݱ�ǩ���ݼ����Ũ��������ʵ���Ũ��Ϊc(H2SO4)�� mol��L��1��19.8mol��L��1�������к͵ζ����Ũ�����Ũ��Ϊc(H2SO4)��mol��L��1��17.8mol��L��1<19.8mol��L��1
(2)ͨ�����������������������ע������ˮ����û��������ˮ��������ע������ˮ���ظ����ֱ������ϴ��Ϊֹ�����ϲ���Һ�еμ��Ȼ���(�����ᱵ��)��Һ����������ɫ��������˵��SOû����ȫ����������������ɫ��������˵��SO����ȫ����
(3)ǿ��ǿ��ĵζ�����ʹ�÷�̪�����ȣ�
(4)����δ��ϴ������NaOHŨ�ȼ�С������������ⶨ���ƫ�ߣ��ڵζ�ǰ�������ݣ�������ռ�������ȷ�����ʲ���ȷ������Ϊ��ȷ������û��Ӱ�죬������������ӣ������ӵĽǶ��Ƿ�һ����ͬ������ȷ�����ݵζ�Ǯ���ӣ��ζ����ӣ������ƫС���ⶨŨ��ƫС���ʴ�Ϊ�ڢܣ�
(5)���������ȥ���������(VNaOH��39.65 mL)���ɵã���35.60 mL�����ݱ�ǩ���ݼ����Ũ��������ʵ���Ũ��Ϊc(H2SO4)�� mol��L��1��19.8mol��L��1�������к͵ζ����Ũ�����Ũ��Ϊc(H2SO4)��mol��L��1��17.8mol��L��1<19.8mol��L��1

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ