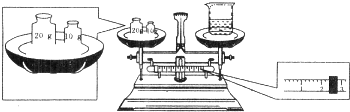
��Ŀ����
ʵ�����õ�����CuSO4?5H2O������490mL0.1mol/LCuSO4��Һ��ʵ�ʲ��������У�
��1�����������������Ϊ______g
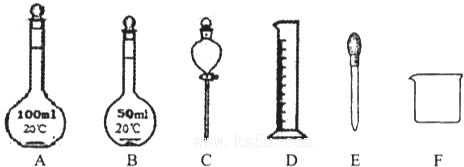
��2��������Һʱ���������У�
A����ƿB.200mL����ƿC���ձ�D����ͷ�ι�E��ҩ��F��������ƽ
����Ҫ�õ�����______��______������ţ�����ȱ�IJ���������______��______��д���ƣ���
��3�����dz������ƹ��̼���Ϊ���¸����裺A����ȴB������C��ϴ��D������E���ܽ�F��ҡ��G��ת��H��װƿ������ȷ�IJ���˳��Ӧ��______������ţ���
��BEFGCGDHA��BEGACGFDH
��BEAGCGDFH��BEAGCGDHF
��4�����ƹ����У����������ʹ���ƽ��ƫ�ߵ���______������ţ�
�ٶ���ʱ���ӿ̶��߹۲�Һ��ڶ���ʱ���ӿ̶��߹۲�Һ�������ƿʹ��ʱδ����ܶ��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶��ߢ���Һʱδϴ���ձ��Ͳ������������岿��ʧˮ��
��1�����������������Ϊ______g
��2��������Һʱ���������У�
A����ƿB.200mL����ƿC���ձ�D����ͷ�ι�E��ҩ��F��������ƽ
����Ҫ�õ�����______��______������ţ�����ȱ�IJ���������______��______��д���ƣ���
��3�����dz������ƹ��̼���Ϊ���¸����裺A����ȴB������C��ϴ��D������E���ܽ�F��ҡ��G��ת��H��װƿ������ȷ�IJ���˳��Ӧ��______������ţ���
��BEFGCGDHA��BEGACGFDH
��BEAGCGDFH��BEAGCGDHF
��4�����ƹ����У����������ʹ���ƽ��ƫ�ߵ���______������ţ�
�ٶ���ʱ���ӿ̶��߹۲�Һ��ڶ���ʱ���ӿ̶��߹۲�Һ�������ƿʹ��ʱδ����ܶ��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶��ߢ���Һʱδϴ���ձ��Ͳ������������岿��ʧˮ��
��1����Ҫ����ͭ�����ʵ�����������ͭ��������ʵ�����ʵ����û��490mL����ƿ��Ӧѡ��500mL����ƿ������500mL0.1mol/LCuSO4��Һ����Ҫ����ͭ���������Ϊ0.1L��0.5mol/L��250g/mol=12.5g���ʴ�Ϊ��12.5��
��2������˳���ǣ�������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��һ������ƽ�������õ�ҩ�ף����������ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�
������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
�����Բ���Ҫ�������У�A����ƿ��B.200mL����ƿ��
��ȱ�ٵ�����Ϊ��������500mL����ƿ��
�ʴ�Ϊ��A��B���ձ�����������
��3���ɣ�2���е�ʵ����������֪����ȷ�Ĵ���˳��ΪBEAGCGDFH����ѡ���ۣ�
��4���ٶ���ʱ���ӿ̶��߹۲�Һ�棬����������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
�ڶ���ʱ���ӿ̶��߹۲�Һ�棬����������Һ�����ƫ��������Һ��Ũ��ƫ�ͣ�
����Һ�������ˮ���ݣ�����ƿʹ��ʱδ�����������ҺŨ����Ӱ�죻
�ܶ��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ�������Һ������ƿ����ƿ��֮�䣬�ټ�����ˮ�����̶��ߣ�����������Һ�����ƫ����Һ��Ũ��ƫ�ͣ�
����Һʱδϴ���ձ��Ͳ���������������ƿ������ͭ��������С��������ҺŨ��ƫ�ͣ�
�������岿��ʧˮ������������ͭ�ĺ�������ʵ�ʳ�ȡ�ľ���������ͭ������ƫ��������ҺŨ��ƫ��
��ѡ���٢ޣ�
��2������˳���ǣ�������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��һ������ƽ�������õ�ҩ�ף����������ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�
������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
�����Բ���Ҫ�������У�A����ƿ��B.200mL����ƿ��
��ȱ�ٵ�����Ϊ��������500mL����ƿ��
�ʴ�Ϊ��A��B���ձ�����������
��3���ɣ�2���е�ʵ����������֪����ȷ�Ĵ���˳��ΪBEAGCGDFH����ѡ���ۣ�
��4���ٶ���ʱ���ӿ̶��߹۲�Һ�棬����������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
�ڶ���ʱ���ӿ̶��߹۲�Һ�棬����������Һ�����ƫ��������Һ��Ũ��ƫ�ͣ�
����Һ�������ˮ���ݣ�����ƿʹ��ʱδ�����������ҺŨ����Ӱ�죻
�ܶ��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ�������Һ������ƿ����ƿ��֮�䣬�ټ�����ˮ�����̶��ߣ�����������Һ�����ƫ����Һ��Ũ��ƫ�ͣ�
����Һʱδϴ���ձ��Ͳ���������������ƿ������ͭ��������С��������ҺŨ��ƫ�ͣ�
�������岿��ʧˮ������������ͭ�ĺ�������ʵ�ʳ�ȡ�ľ���������ͭ������ƫ��������ҺŨ��ƫ��
��ѡ���٢ޣ�

��ϰ��ϵ�д�
�����Ŀ