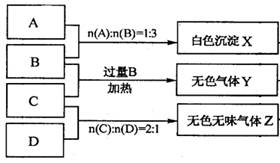
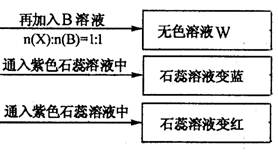
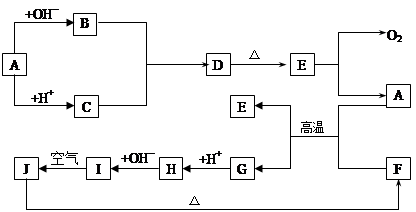��Ŀ����
(10��)
��A���ʵĻ�ѧʽΪM(OH)2����������ˮ�Ƴ�ϡ��Һ������Һ�����ԣ�����Һ�д��ڣ�
M2+ + 2OH- M(OH)2
M(OH)2 2H+ + MO22-
2H+ + MO22-
�ش�������Ŀһ���á�������С�������䡱���
��1���������������������£�25Coʱ��������ˮ�м���A���ʺ������ǰ�Ƚϣ�
����ˮ�������C(OH-) ��C(H+) ��ˮ�ĵ���� ��Kw
��2����4�֣�25Coʱ����A��ϡ��Һ�м����������ռ���塣
��ˮ�ĵ���� ����Һ��pH
��.(2��)�����£��ס�����ƿ��ˮ��Ũ�ȷֱ�Ϊ1mol/L��0.1mol/L,��ס�����ƿ��ˮ��C(OH-)֮��Ϊ 10 (����ڡ����ڻ�С��)
(10��)
��(1)
��C(OH-)��� ��C(H+)������ˮ�ĵ������С ��Kw ����
��2��
��ˮ�ĵ���� ���� ����Һ��pH����
��.(2��)C(OH-)֮��Ϊ ���� 10 (����ڡ����ڻ�С��)
����

��ϰ��ϵ�д�
�����Ŀ