��Ŀ����
(10��)��֪AΪ����������X��YΪ�����ǽ�����X��E��F��G������Ϊ���壬CΪҺ�壬B��һ���Σ����ȼ��ֽ⣬�ڹ�ũҵ��������;�Ϲ㣨�类����ijЩ��صĵ���ʣ�������A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й�����֮���ת����ϵ����ͼ��
��ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ��
����д���пհף�
��1����Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������䷴Ӧ����ʽΪ��
��2����D��Һ�Ʊ�D����ˮ����ġ�����a��Ϊ ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����������
��4����Ӧ�ݵĻ�ѧ����Ϊ ����������
��5��ԭ��ط�Ӧ���������ĵ缫��ӦΪ ��
��1��2Mg+CO2="2MgO+C;" ��2����D��Һ��HCl���������ɣ�
��3����4NH3+5O2  4NO+6H2O; ��4����C+4HNO3��Ũ��
4NO+6H2O; ��4����C+4HNO3��Ũ��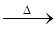 CO2��+4NO2��+2H2O
CO2��+4NO2��+2H2O
��5��2NH4++2e- = 2NH3��+H2��
����

��ϰ��ϵ�д�
�����Ŀ

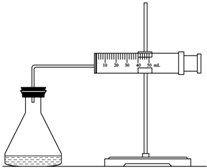 ʵ������H2O2�ֽⷴӦ��ȡ����ʱ������������Լӿ췴Ӧ���ʣ�ij�о���ѧϰС��Ϊ�о�����FeCl3������O2�������ʵ�Ӱ�죬�������������ʵ�鷽�������±������������������Լ���һ�������Ϻ���з�Ӧ��
ʵ������H2O2�ֽⷴӦ��ȡ����ʱ������������Լӿ췴Ӧ���ʣ�ij�о���ѧϰС��Ϊ�о�����FeCl3������O2�������ʵ�Ӱ�죬�������������ʵ�鷽�������±������������������Լ���һ�������Ϻ���з�Ӧ��
 ��
�� �ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
�ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ CO(g) +3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
CO(g) +3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ�������� �ֱ�Ϊ
�ֱ�Ϊ ��
�� ����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��

 Cr2O72������ɫ��+H2O
Cr2O72������ɫ��+H2O