��Ŀ����
(10�֣���A��B��C��D�������ӻ����������ǵ����ӷֱ�Ϊ��
�����ӣ�Na����Al3����NH4+�� �����ӣ�OH����NO3����CO32����HSO4��
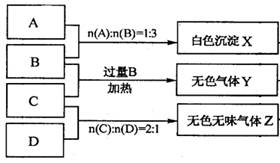
Ϊ�������ֻ����ijѧ���ֱ�ȡ�������������Һ�����ΪA��B��C��D����ʵ�顣ʵ����̺ͼ�¼����ͼ��ʾ������������ȥ��
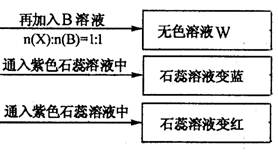
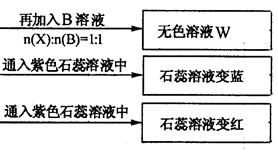
(1) Y��Z�Ļ�ѧʽ�ֱ�Ϊ��Y ��Z
(2)д��ָ����Ӧ�����ӷ���ʽ��
�ټ��������£�C�����B��Ӧ��
��D��Һ�������Ե�ԭ����(�����ӷ���ʽ��ʾ)
(3)�����ʵ���Ũ�ȵ�A��B��C��D��ҺpH�ɴ�С��˳����(�û�ѧʽ��ʾ)
(4)��B��C��ϡ��Һ��Ϻ�(������)��Һ�����ԣ�����Һ������Ũ�ȴӴ�С��˳���ǣ�
(1)NH3(1��) CO2(1��)
(2)��NH4++H++2OH-��NH3��+2H2O(2��)
��CO32-+H2O HCO3-+OH-
(2��)
HCO3-+OH-
(2��)
(3) NaOH>Na2CO3>Al(NO3)3>NH4HSO4(2��)
(4) c(Na+)>c(SO42-)>c(NH4+)>c(H+)=c(OH-)(2��)
����������1����ʹ��ɫ��ʯ����Һ����ɫ���ǰ�������ʹ��ɫʯ����Һ�Ժ�ɫ�����������壬���Ը��������֪Y�ǰ�����Z��CO2��
��2����ɫ����X������B�У���˳����������������Ը��ݷ�Ӧ�����ʵ���֮�ȿ�֪��A����������B���������ơ�C��������泥�D��̼���ơ�
�ټ��������£�C�����B��Ӧ�ķ���ʽΪNH4++H++2OH-��NH3��+2H2O��
��̼������ǿ�������Σ�CO32��ˮ���Լ��ԣ�����ʽΪCO32-+H2O HCO3-+OH-��
HCO3-+OH-��
��3������������ǿ�������ǿ��̼����ˮ���Լ��ԣ�������ˮ�������ԣ�������淋���������������ԣ����Ե����ʵ���Ũ�ȵ�A��B��C��D��ҺpH�ɴ�С��˳����NaOH>Na2CO3>Al(NO3)3>NH4HSO4��
��4��B��C��ϡ��Һ��Ϻ�(������)��Һ�����ԣ�����Һ�к��е������������ơ�����狀Ͱ�ˮ��������Һ������Ũ�ȴӴ�С��˳���� c(Na+)>c(SO42-)>c(NH4+)>c(H+)=c(OH-)��