��Ŀ����
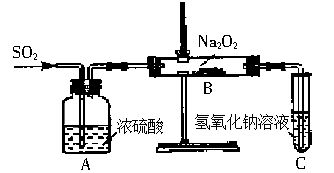
������ʵ��С���ͬѧΪ̽�������������������ķ�Ӧ����������ͼ��ʾ��װ�ý���ʵ�飮ͨ��SO2���壬�������ǵ�ľ�������Թ�C�У�ľ����ȼ����ش��������⣺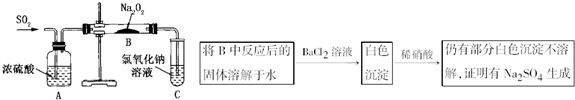
��1����1С��ͬѧ��ΪNa2O2��SO2��Ӧ������Na2SO3��O2���÷�Ӧ�Ļ�ѧ����ʽ��
��2�������һ��ʵ�鷽��֤��Na2O2��SO2��Ӧ���ɵİ�ɫ�����к���Na2SO3��
��3����2С��ͬѧ��ΪNa2O2��SO2��Ӧ��������Na2SO3��O2�⣬����Na2SO4���ɣ�Ϊ�����Ƿ���Na2SO4���ɣ�������������·��������������Ƿ������

��1����1С��ͬѧ��ΪNa2O2��SO2��Ӧ������Na2SO3��O2���÷�Ӧ�Ļ�ѧ����ʽ��
2Na2O2+2SO2�T2Na2SO3+O2
2Na2O2+2SO2�T2Na2SO3+O2
��2�������һ��ʵ�鷽��֤��Na2O2��SO2��Ӧ���ɵİ�ɫ�����к���Na2SO3��
ȡ��ɫ���壬��ϡ���ᣬ������ʹƷ����Һ��ɫ������
ȡ��ɫ���壬��ϡ���ᣬ������ʹƷ����Һ��ɫ������
����3����2С��ͬѧ��ΪNa2O2��SO2��Ӧ��������Na2SO3��O2�⣬����Na2SO4���ɣ�Ϊ�����Ƿ���Na2SO4���ɣ�������������·��������������Ƿ������
������
������
�����Ҫ˵���������ɣ���ϡ�����ܽ������ᱵ����Ϊ���ᱵ
ϡ�����ܽ������ᱵ����Ϊ���ᱵ
���������Ӧ��Ĺ����л�����Na2O2Ҳ�ɽ������ᱵ����Ϊ���ᱵ��
�����Ӧ��Ĺ����л�����Na2O2Ҳ�ɽ������ᱵ����Ϊ���ᱵ��
����������1������CO2��Na2O2�ķ�Ӧ����ʽ����д��
��2������SO32-�ļ��鷽����
��3�����ݼ���SO42-�ķ�����ͬʱ����SO32-�ĸ��ţ�
��2������SO32-�ļ��鷽����
��3�����ݼ���SO42-�ķ�����ͬʱ����SO32-�ĸ��ţ�
����⣺��1����CO2��Na2O2�ķ�Ӧ��������Na2SO3��O2����ѧ����ʽΪ2Na2O2+2SO2�T2Na2SO3+O2���ʴ�Ϊ��2Na2O2+2SO2�T2Na2SO3+O2��
��2��Ҫ֤����ɫ�����к���Na2SO3ֻ����������SO32-�Ϳ����ˣ�ȡ������ɫ���壬����ϡ�����ϡ���ᣬ������ʹƷ����Һ��ɫ�����壬��֤�����ɵİ�ɫ�����к���Na2SO3��
�ʴ�Ϊ��ȡ��ɫ���壬��ϡ���ᣬ������ʹƷ����Һ��ɫ�����壻
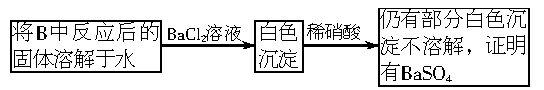
��3���ڼ����Ƿ���Na2SO4����ʱ��Ҫ���ǵ�Na2SO3�ĸ��ţ���ΪSO32-�н�ǿ�Ļ�ԭ�ԣ��ɱ����ᡢ�������Ƶ�������SO4���ʴ�Ϊ����������ϡ�����ܽ������ᱵ����Ϊ���ᱵ�������Ӧ��Ĺ����л�����Na2O2Ҳ�ɽ������ᱵ����Ϊ���ᱵ��
��2��Ҫ֤����ɫ�����к���Na2SO3ֻ����������SO32-�Ϳ����ˣ�ȡ������ɫ���壬����ϡ�����ϡ���ᣬ������ʹƷ����Һ��ɫ�����壬��֤�����ɵİ�ɫ�����к���Na2SO3��
�ʴ�Ϊ��ȡ��ɫ���壬��ϡ���ᣬ������ʹƷ����Һ��ɫ�����壻
��3���ڼ����Ƿ���Na2SO4����ʱ��Ҫ���ǵ�Na2SO3�ĸ��ţ���ΪSO32-�н�ǿ�Ļ�ԭ�ԣ��ɱ����ᡢ�������Ƶ�������SO4���ʴ�Ϊ����������ϡ�����ܽ������ᱵ����Ϊ���ᱵ�������Ӧ��Ĺ����л�����Na2O2Ҳ�ɽ������ᱵ����Ϊ���ᱵ��
����������Ϊ�о������⣬��̽�������������������ķ�ӦΪ���У�����֪ʶ��Ǩ����������ƺ�����ʵ�鷽����������

��ϰ��ϵ�д�
�����Ŀ
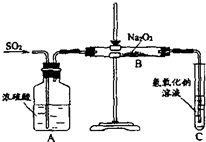


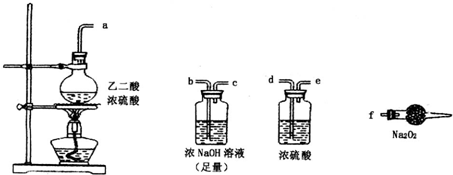
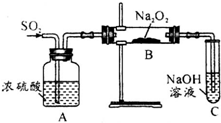 ������ʵ��С���ͬѧ̽��һ����̼������������������Ƶķ�Ӧ����ش��������⣺
������ʵ��С���ͬѧ̽��һ����̼������������������Ƶķ�Ӧ����ش��������⣺
 0
0