��Ŀ����
��1����һС����Ʒ������������£�

��д��n ��OH-����n ��Ca2+����n ��Na+���Ĺ�ϵ��n��Na+��=
����n ��Na+��=x mol��n ��Ca2+��=y mol����������
| ��ʯ�ҵ� ������� |
NaOH��CaO | NaOH��CaO�� Ca ��OH��2 |
NaOH�� Ca ��OH��2 |
NaOH�� Ca ��OH��2��H2O |
| n ��Na+����n ��Ca2+�� �Ĺ�ϵ |
40x+56y=4 | 40x+56y��4.0��40x+74y 40x+56y��4.0��40x+74y |
40x+74y=4.0 40x+74y=4.0 |
40x+74y��4.0 40x+74y��4.0 |
������������·������ó�������ݣ�ȡ���ۼ�ʯ��4.0g����250��ʱ���������أ���ù�������������0.42g��ʣ�������580��ʱ�������������أ����������ּ�����0.75g����ͨ������ȷ���ü�ʯ�Ҹ��ɷֵ�����������
�����ݼ�ʯ�ҵ�������ʽ�ϼ�ֵ�����жϵõ������Ӻ����ӵ����ʵ����Ĺ�ϵ��
n ��Na+��=x mol��n ��Ca2+��=y mol�������������ƺ������ƣ��������ƺ��������ƻ�����������ϼ�ֵ�����õ���
��2��Ca��OH��2��250��ʱ���ֽ⡢NaOH��580��ʱ���ֽ⣬ȡ���ۼ�ʯ��4.0g����250��ʱ���������أ���ù�������������0.42g��Ϊˮ��ʣ�������580��ʱ�������������أ����������ּ�����0.75g��Ϊ���ٵ��������Ʒֽ����ɵ�ˮ������õ��������Ƶ�������ʣ���������Ϊ��������������
�ʴ�Ϊ��n��OH-��-2n��Ca2+����
�ڼ�ʯ����NaOH��CaO��n ��Na+����n ��Ca2+���Ĺ�ϵ 40x+56y=4��
��ʯ����NaOH��CaO��Ca ��OH��2��n ��Na+����n ��Ca2+���Ĺ�ϵ 40x+56y��4.0��40x+74y
��ʯ����NaOH��Ca ��OH��2��n ��Na+����n ��Ca2+���Ĺ�ϵ 40x+74y=4.0��
��ʯ����NaOH��CaO��Ca ��OH��2��H2O��n ��Na+����n ��Ca2+���Ĺ�ϵ 40x+74y��4.0
�ʴ�Ϊ��
| ��ʯ�ҵ� ������� |
NaOH��CaO | NaOH��CaO�� Ca ��OH��2 |
NaOH�� Ca ��OH��2 |
NaOH�� Ca ��OH��2��H2O |
| n ��Na+����n ��Ca2+�� �Ĺ�ϵ |
40x+56y=4 | 40x+56y��4.0��40x+74y | 40x+74y=4.0 | 40x+74y��4.0 |
| 0.42g |
| 4.0g |
| 0.75g |
| 18g/mol |
| ||
| 4g |
| 0.497g |
| 4.0g |
�𣺸ü�ʯ�Ҹ��ɷֵ�����������H2O%=10.5%�� Ca��OH��2%=77.1%�� NaOH%=12.4%��

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
| �۵�/�� | �е�/�� |
���Ȼ��� | -122 | 76 |
���Ȼ��� | 148 | 200 ��ֽ� |
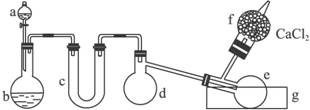
��1����Ũ���ᡢŨ���ᡢ���ס��������̡��������Ƶ����ʹ�ѡ�ã�a��b��Ӧ��װ����Լ��ֱ��ǣ�a________��b________��
��2��________����������Ӧ����ĸ��������Ҫ���ȡ�
��3�����ɵ����Ȼ�����������ƿe���ռ���Ϊ��֤���Ȼ�������������Ӧ��ˮ��g�м���________��
��4�����Ȼ�������ˮ����ǿ�ҷ�Ӧ������������ը������d��e������װ�����е����ʶ����ܺ���ˮ�֡�Ϊ��ȥ�����е�ˮ�֣�c����װ�����������е�________������ĸ����
A����ʯ�� B��Ũ���� C����ˮ�Ȼ���
��5�������Ͱ���Ӧ�ų��������ȣ�Ϊʹ��֧��ƿd������ֲ����ȶ�ը�ѣ�ʵ�鿪ʼǰӦ����ƿ�ĵײ�������________��
��6��ʵ���ҵİ��ױ�����ˮ�У�ȡ����������ֽ���ɱ���ˮ�֣�������ˮ�ƾ���Ƭ�̣��ٽ���������Ƭ�̼�����ȫ��ȥˮ�֡���֪ˮ��ƾ����ܣ��ƾ������ѻ��ܣ������������ɳ�ȥˮ��ԭ����________________________________________________________��
��7��Ϊ��ֹ������Ⱦ������װ��ĩ�˵�������������ã�����ĸ��________���о���������
A��NaOH��Һ B��Ca(OH)2��Һ C������ʳ��ˮ
��ͼ1-3��ʾװ�ã��ƾ��ơ�����̨��δ��������ȡ���Ȼ��ס��ھ�֧��ƿd�з����������ף�������Ѹ�ٶ��ֲ���ϵ�ͨ���֧��ƿ�У���������ͻᷢ����Ӧ���������档���Ȼ������Ȼ��������������±���
| �۵�/�� | �е�/�� | |
| ���Ȼ��� | -122 | 76 |
| ���Ȼ��� | 148 | 200 ��ֽ� |
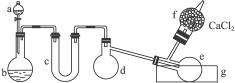
ͼ1-3
��1����Ũ���ᡢŨ���ᡢ���ס��������̡��������Ƶ����ʹ�ѡ�ã�a��b��Ӧ��װ����Լ��ֱ��ǣ�a________��b________��
��2��________����������Ӧ����ĸ��������Ҫ���ȡ�
��3�����ɵ����Ȼ�����������ƿe���ռ���Ϊ��֤���Ȼ�������������Ӧ��ˮ��g�м���________��
��4�����Ȼ�������ˮ����ǿ�ҷ�Ӧ������������ը������d��e������װ�����е����ʶ����ܺ���ˮ�֡�Ϊ��ȥ�����е�ˮ�֣�c����װ�����������е�________������ĸ����
A����ʯ�� B��Ũ���� C����ˮ�Ȼ���
��5�������Ͱ���Ӧ�ų��������ȣ�Ϊʹ��֧��ƿd������ֲ����ȶ�ը�ѣ�ʵ�鿪ʼǰӦ����ƿ�ĵײ�������________��
��6��ʵ���ҵİ��ױ�����ˮ�У�ȡ����������ֽ���ɱ���ˮ�֣�������ˮ�ƾ���Ƭ�̣��ٽ���������Ƭ�̼�����ȫ��ȥˮ�֡���֪ˮ��ƾ����ܣ��ƾ������ѻ��ܣ������������ɳ�ȥˮ��ԭ����________________________________________________________��
��7��Ϊ��ֹ������Ⱦ������װ��ĩ�˵�������������ã�����ĸ��________���о���������
A��NaOH��Һ B��Ca(OH)2��Һ C������ʳ��ˮ
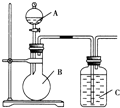 ijͬѧ�����ͼװ�ã��о��ǽ���Ԫ�����ʱ仯���ɣ�
ijͬѧ�����ͼװ�ã��о��ǽ���Ԫ�����ʱ仯���ɣ�