��Ŀ����
��16�֣���ˮ��Դ�����þ��й���ǰ������ˮ����Ҫ���ӵĺ������£�
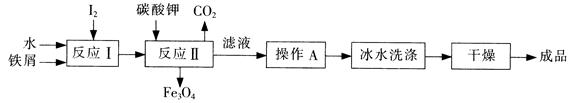
��������������ˮʾ��ͼ��ͼ��ʾ��������������
���ӽ���Ĥ������������������ͨ����

��������Ҫ�缫��Ӧʽ�� ��
����������������������ɫ��������ɷ��� ��CaCO3��
����CaCO3�����ӷ���ʽ�� ��
�۵�ˮ�ij���Ϊ ���a������b����c������
��2�����ú�ˮ������ȡ���þ����ȡ�������£�
����ȡ��Ĺ����У�����2��Br����Br2ת����Ŀ���� ���������з�����Ӧ�����ӷ���ʽ�� ������ͨ������Ŀ���� ��
�ڴ�MgCl2��Һ�еõ�MgCl2��6H2O�������Ҫ������ �����ˡ�ϴ�ӡ����
�������������̣�����10 m3��ˮ�е���Ԫ��ת��Ϊ��ҵ�壬������Ҫ��״����Cl2�����Ϊ
L������Cl2�ܽ�,������ԭ��������80����
| �ɷ� | ����/(mg L��1) | �ɷ� | ����/(mg L��1) |
| Cl�� | 18980 | Ca2+ | 400 |
| Na+ | 10560 | HCO3- | 142 |
| SO42 | 2560 | Br�� | 64 |
| Mg2+ | 1272 | | |
��������������ˮʾ��ͼ��ͼ��ʾ��������������
���ӽ���Ĥ������������������ͨ����

��������Ҫ�缫��Ӧʽ�� ��
����������������������ɫ��������ɷ��� ��CaCO3��
����CaCO3�����ӷ���ʽ�� ��
�۵�ˮ�ij���Ϊ ���a������b����c������
��2�����ú�ˮ������ȡ���þ����ȡ�������£�

����ȡ��Ĺ����У�����2��Br����Br2ת����Ŀ���� ���������з�����Ӧ�����ӷ���ʽ�� ������ͨ������Ŀ���� ��
�ڴ�MgCl2��Һ�еõ�MgCl2��6H2O�������Ҫ������ �����ˡ�ϴ�ӡ����
�������������̣�����10 m3��ˮ�е���Ԫ��ת��Ϊ��ҵ�壬������Ҫ��״����Cl2�����Ϊ
L������Cl2�ܽ�,������ԭ��������80����
��1���٣�2�֣�2Cl��-2e��=Cl2��
�ڣ�2�֣�Mg(OH)2 ��2�֣�Ca2++ OH��+HCO3��=CaCO3��+H2O
�ۣ�2�֣�b
��2���٣�1�֣�����Ԫ�ؽ��и��� ��2�֣�SO2+Br2+2H2O=4H++2Br��+SO42��
��2�֣�ͨ�������Br2��������
�ڣ�1�֣�����Ũ������ȴ�ᾧ
�ۣ�2�֣�179.2
���������
��1���ٸ��ݺ�ˮ�����ӳɷֱ��ͷŵ�˳���֪�������ķ�Ӧʽ2Cl��-2e��=Cl2�����������ķ�Ӧʽ�ǣ�2H+ + 2e- =H2 ,��������ˮ��������ģ������ӷŵ���̴ٽ���ˮ�ĵ��룬������ΧOH-Ũ�����ӣ���þ���ӽ������Mg(OH)2������ͬʱ���ɵ�OH-��HCO3-����ˮ��CO32-��CO32-��Ca2+��Ӧ���ɳ�������ˮ�ij��ڿ��Դ��������ӵ��ƶ�������
��2�����ں�ˮ�е������Ӳ����ߣ�����2��ת����Ŀ����Ϊ�����Ũ�ȣ����Ǹ��������������ҵ��������������������к��е������������Ӧ�����ǿ�����ԻὫ����������������������Һ���������������ʽ���ڣ��嵥�ʱ���ԭ�����������ӣ�д����ʽʱ������дSO2+ Br2��2Br��+SO42���ٸ��ݵ�ԭ���غ�͵���غ㣬�ڱ߲���ˮ���ұ߲��������ӣ���ƽ�Ϳ����ˣ���Ϊ���ӷ���ͨ�����������������ã�����Ҫ�ǽ�������������
�����⡰����Һ�����塱����Ҫ������Ũ��������ȴ�ᾧ��
�����ϱ���֪��Br-�ĺ���Ϊ64mg/L,������10m3��ˮ�����е������ӵ����ʵ���Ϊ��
10��1000L��64��10-3g/L��80g/mol=8mol,����2Br- + Cl2 = Br2 +2Cl-���м���õ�Cl2�ڱ���µ����ӦΪ89.6L��������Ҫ����2��Br����Br2ת������������Ҫ��179.2L��

��ϰ��ϵ�д�
 ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д�
�����Ŀ
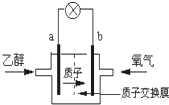
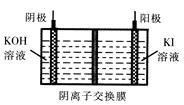
 ��������ΪSO42����a��b��Ϊ���Ե缫�����ʱ�Ҳ���Һ��ɫ����ɫ��Ϊ��ɫ�����жԴ˵��������ȷ����
��������ΪSO42����a��b��Ϊ���Ե缫�����ʱ�Ҳ���Һ��ɫ����ɫ��Ϊ��ɫ�����жԴ˵��������ȷ����
 Al2O3 + 3H2�����������У������ж���ȷ���ǣ�������
Al2O3 + 3H2�����������У������ж���ȷ���ǣ�������