��Ŀ����
��2012?�ӱ���ģ�⣩��ͼ1Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����ӦԪ�أ�
��ش��������⣺
��1��Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ����
A������������
B�����ں��м��Լ��ķǼ��Է���
C��ֻ����4���Ҽ���1���м�
D�����⻯�������Ԫ�آ�ԭ�Ӳ���sp2�ӻ�
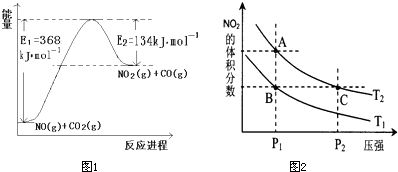
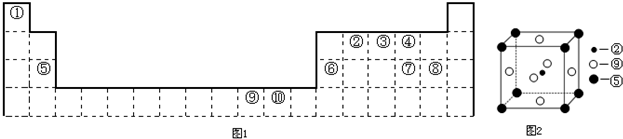
��2����ѧ���֣��ڡ��ݡ�������Ԫ�ص�ԭ���γɵľ�����г����ԣ��侧���Ľṹ��ͼ2��ʾ��ͼ�Тڡ��ݡ���ֱ�λ�ھ��������ġ����㡢���ģ�����û�����Ļ�ѧʽΪ
��3��a���ȽϢڡ��ۡ�������Ԫ�ص�һ�����ܴ�С����С�������е�˳��Ϊ
b���ȽϢܡ��ޡ��ߡ�������Ԫ�صĵ縺�Դ�С���ɴ�С���е�˳��Ϊ
��4��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1����Ԫ����Ԫ�آ��γɵ�18���ӵ�X���ӵĽṹʽΪ
 ����Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y����������Y����ͨ��ʢ�к���Ԫ�ص���������Һ�У���Ӧ�����е�ʵ������Ϊ��
����Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y����������Y����ͨ��ʢ�к���Ԫ�ص���������Һ�У���Ӧ�����е�ʵ������Ϊ��
��5����Ԫ�ص��ʾ��������������壬�������ÿ����5����ԭ�ӽ��ܶ�������ԭ�Ӱ뾶Ϊd cm���ý������ܶ�Ϊ
g?cm-3�����ý���ԭ�ӵ�����Ϊa g��

��ش��������⣺
��1��Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ����
BD
BD
��A������������
B�����ں��м��Լ��ķǼ��Է���
C��ֻ����4���Ҽ���1���м�
D�����⻯�������Ԫ�آ�ԭ�Ӳ���sp2�ӻ�
��2����ѧ���֣��ڡ��ݡ�������Ԫ�ص�ԭ���γɵľ�����г����ԣ��侧���Ľṹ��ͼ2��ʾ��ͼ�Тڡ��ݡ���ֱ�λ�ھ��������ġ����㡢���ģ�����û�����Ļ�ѧʽΪ
MgNi3C
MgNi3C
���ö�Ӧ��Ԫ�ط��ű�ʾ������3��a���ȽϢڡ��ۡ�������Ԫ�ص�һ�����ܴ�С����С�������е�˳��Ϊ
C��O��N
C��O��N
����Ԫ�ط��ű�ʾ����b���ȽϢܡ��ޡ��ߡ�������Ԫ�صĵ縺�Դ�С���ɴ�С���е�˳��Ϊ
O��Cl��S��Al
O��Cl��S��Al
����Ԫ�ط��ű�ʾ������4��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1����Ԫ����Ԫ�آ��γɵ�18���ӵ�X���ӵĽṹʽΪ


��������ɫ������������ܽ⣬��Һ������ɫ
��������ɫ������������ܽ⣬��Һ������ɫ
����Ӧ�����е����ӷ���ʽΪCu2++2 NH3?H2O=Cu��OH��2��+2 NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-
Cu2++2 NH3?H2O=Cu��OH��2��+2 NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-
����5����Ԫ�ص��ʾ��������������壬�������ÿ����5����ԭ�ӽ��ܶ�������ԭ�Ӱ뾶Ϊd cm���ý������ܶ�Ϊ
| ||
| 8d3 |
| ||
| 8d3 |
��������Ԫ���������ڱ��е�λ�ÿ�֪��ΪH����ΪC����ΪN����ΪO����ΪMg����ΪAl����ΪS����ΪCl����ΪNi����ΪCu��
��1��Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־��Ϊ��ϩ��
��2�����þ�̯�����㣻
��3��a��ͬ���ڴ����ң�Ԫ�صĵ�һ������������ע��Nԭ�������Ϊ�������������
b������ͬ���ڴ����ҵ縺��������ͬ������ϵ��µ縺����С�жϣ�
��4��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1��ӦΪ2s22p3��ӦΪNԪ�أ���Ԫ�آ��γɵ�18���ӵ�X����ΪN2H4����Ԫ�آ��γ�10���ӵ��������YΪNH3������ͨ��ͭ����Һ�У���������ɫ������������Ӧ��������
��5�����þ�̯�����㾧���к��е�Cuԭ�Ӹ�����������������Ϧ�=
���㣮
��1��Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־��Ϊ��ϩ��
��2�����þ�̯�����㣻
��3��a��ͬ���ڴ����ң�Ԫ�صĵ�һ������������ע��Nԭ�������Ϊ�������������
b������ͬ���ڴ����ҵ縺��������ͬ������ϵ��µ縺����С�жϣ�
��4��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1��ӦΪ2s22p3��ӦΪNԪ�أ���Ԫ�آ��γɵ�18���ӵ�X����ΪN2H4����Ԫ�آ��γ�10���ӵ��������YΪNH3������ͨ��ͭ����Һ�У���������ɫ������������Ӧ��������
��5�����þ�̯�����㾧���к��е�Cuԭ�Ӹ�����������������Ϧ�=
| m |
| V |
����⣺��Ԫ���������ڱ��е�λ�ÿ�֪��ΪH����ΪC����ΪN����ΪO����ΪMg����ΪAl����ΪS����ΪCl����ΪNi����ΪCu��
��1��Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־��Ϊ��ϩ���ṹʽΪ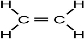 ����֪�����к���C=C�Ǽ��Լ���C-H���Լ���Ϊƽ���η��ӣ�����5���Ҽ���1���м���ÿ��C�γ�3���Ҽ���Ϊsp2�ӻ���
����֪�����к���C=C�Ǽ��Լ���C-H���Լ���Ϊƽ���η��ӣ�����5���Ҽ���1���м���ÿ��C�γ�3���Ҽ���Ϊsp2�ӻ���
�ʴ�Ϊ��BD��
��2�������У�����Mg����Ϊ8��
=1��Ni����Ϊ6��
=3��Nԭ�Ӹ���Ϊ1����ѧʽΪMgNi3C��
�ʴ�Ϊ��MgNi3C��
��3��a��ͬ���ڴ����ң�Ԫ�صĵ�һ������������Nԭ�������Ϊ������ṹ����һ�����ܴ�������ԭ�ӵĵ�һ�����ܣ���˳��ΪC��O��N��
�ʴ�Ϊ��C��O��N��
b��ͬ���ڴ����ҵ縺��������ͬ������ϵ��µ縺����С���縺��˳��ΪO��Cl��S��Al���ʴ�Ϊ��O��Cl��S��Al��
��4��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1��ӦΪ2s22p3��ӦΪNԪ�أ���Ԫ�آ��γɵ�18���ӵ�X����ΪN2H4���ṹʽΪ ����Ԫ�آ��γ�10���ӵ��������YΪNH3������ͨ��ͭ����Һ�У���������ɫ������������Ӧ����������Ӧ�����ӷ���ʽΪCu2++2 NH3?H2O=Cu��OH��2��+2 NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
����Ԫ�آ��γ�10���ӵ��������YΪNH3������ͨ��ͭ����Һ�У���������ɫ������������Ӧ����������Ӧ�����ӷ���ʽΪCu2++2 NH3?H2O=Cu��OH��2��+2 NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
�ʴ�Ϊ�� ����������ɫ������������ܽ⣬��Һ������ɫ��Cu2++2 NH3?H2O=Cu��OH��2��+2 NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
����������ɫ������������ܽ⣬��Һ������ɫ��Cu2++2 NH3?H2O=Cu��OH��2��+2 NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
��5��ͭ��������������������ÿ�����к��е�ԭ�Ӹ���=8��
+6��
=4��
ÿ��ͭ�����к���4��ԭ�ӣ�ԭ�Ӱ뾶Ϊd cm���������Ϊ��2
��3cm3������������Ϊ4a��
���=
=
��g?cm-3����
�ʴ�Ϊ��
��
��1��Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־��Ϊ��ϩ���ṹʽΪ
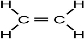 ����֪�����к���C=C�Ǽ��Լ���C-H���Լ���Ϊƽ���η��ӣ�����5���Ҽ���1���м���ÿ��C�γ�3���Ҽ���Ϊsp2�ӻ���
����֪�����к���C=C�Ǽ��Լ���C-H���Լ���Ϊƽ���η��ӣ�����5���Ҽ���1���м���ÿ��C�γ�3���Ҽ���Ϊsp2�ӻ����ʴ�Ϊ��BD��
��2�������У�����Mg����Ϊ8��
| 1 |
| 8 |
| 1 |
| 2 |
�ʴ�Ϊ��MgNi3C��
��3��a��ͬ���ڴ����ң�Ԫ�صĵ�һ������������Nԭ�������Ϊ������ṹ����һ�����ܴ�������ԭ�ӵĵ�һ�����ܣ���˳��ΪC��O��N��
�ʴ�Ϊ��C��O��N��
b��ͬ���ڴ����ҵ縺��������ͬ������ϵ��µ縺����С���縺��˳��ΪO��Cl��S��Al���ʴ�Ϊ��O��Cl��S��Al��
��4��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1��ӦΪ2s22p3��ӦΪNԪ�أ���Ԫ�آ��γɵ�18���ӵ�X����ΪN2H4���ṹʽΪ
 ����Ԫ�آ��γ�10���ӵ��������YΪNH3������ͨ��ͭ����Һ�У���������ɫ������������Ӧ����������Ӧ�����ӷ���ʽΪCu2++2 NH3?H2O=Cu��OH��2��+2 NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
����Ԫ�آ��γ�10���ӵ��������YΪNH3������ͨ��ͭ����Һ�У���������ɫ������������Ӧ����������Ӧ�����ӷ���ʽΪCu2++2 NH3?H2O=Cu��OH��2��+2 NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-���ʴ�Ϊ��
 ����������ɫ������������ܽ⣬��Һ������ɫ��Cu2++2 NH3?H2O=Cu��OH��2��+2 NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
����������ɫ������������ܽ⣬��Һ������ɫ��Cu2++2 NH3?H2O=Cu��OH��2��+2 NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-����5��ͭ��������������������ÿ�����к��е�ԭ�Ӹ���=8��
| 1 |
| 8 |
| 1 |
| 2 |
ÿ��ͭ�����к���4��ԭ�ӣ�ԭ�Ӱ뾶Ϊd cm���������Ϊ��2
| 2d |
���=
| 4a | ||
(2
|
| ||
| 8d3 |
�ʴ�Ϊ��
| ||
| 8d3 |
���������⿼��λ�á��ṹ���ʵ����ϵ�Լ�Ӧ�ã�������ѧ���ķ��������ͼ��������Ŀ��飬Ϊ�߿��������ͣ�ע����վ����ļ��㣬Ϊ������ѵ㣬Ҳ���״��㣬�Ѷ��еȣ�

��ϰ��ϵ�д�
 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
�����Ŀ