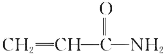��Ŀ����
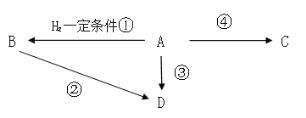
����Ŀ���л�A�Ǻ���һ������ʯ�ͻ�ѧ��ҵ�ķ�չˮƽ��D�ķ���ʽΪC2H5Cl��C��һ�ֳ����ĸ߷ֻ������ش��������⣺
��1��д��A�Ľṹʽ___��
��2��д���ڵķ���ʽ___��;���ڡ��۶����Եõ�D������������;��___��������___��
��3��д������A��B�������ʵ�һ���Լ�___��
��4��д���ܵķ���ʽ___��
��5�����������У����˶�ԱŤ�˻������ʱ����ҽ�漴���˶�Ա�����˴�����Һ��D(�е�12.27��)����ֲ��䶳����������ԭ����___��
���𰸡� CH3CH3+Cl2
CH3CH3+Cl2![]() CH3CH2Cl+HCl �� ���ﵥһ���������� ���Ը��������Һ nCH2=CH2
CH3CH2Cl+HCl �� ���ﵥһ���������� ���Ը��������Һ nCH2=CH2![]()
![]() D�ķе�ϵͣ��ڳ��������ӷ�����Һ̬�����̬ʱ������������ʹ���˲�λ�ֲ��䶳������
D�ķе�ϵͣ��ڳ��������ӷ�����Һ̬�����̬ʱ������������ʹ���˲�λ�ֲ��䶳������
��������
��ϩ�IJ����Ǻ���һ������ʯ�ͻ�ѧ��ҵ�ķ�չˮƽ�ı�־�����л���AΪ��ϩ����ϩ��������һ�������·�Ӧ����B��BΪ���飻����D�ķ���ʽ��D����ͨ����ϩ��HCl�ӳɵõ���Ҳ����ͨ���������������ȡ����Ӧ�õ�����ϩ�ڴ����������¿��Ծ���ϩ������ϩ��һ�ֳ����ĸ߷��ӻ�����ݴ˷�����
��1�����ݷ������л���AΪ��ϩ����ṹʽΪ ��
��
��2����Ӧ��Ϊ�����������ȡ����Ӧ������ʽΪCH3CH3+Cl2![]() CH3CH2Cl+HCl��;���ڡ��۶����Եõ�D������;���۸�Ϊ������ԭ����;���۲��ﵥһ���������ɣ�
CH3CH2Cl+HCl��;���ڡ��۶����Եõ�D������;���۸�Ϊ������ԭ����;���۲��ﵥһ���������ɣ�
��3���л���AΪ��ϩ������̼̼˫�����л���BΪ���飬����̼̼˫������������ߵ��Լ����������Ը��������Һ����ϩ����ʹ���Ը��������Һ��ɫ��
��4����ϩ�ϳɾ���ϩ�ķ���ʽΪ��nCH2=CH2![]()
![]() ��
��
��5��D�ķе�ϵͣ��ڳ��������ӷ�����Һ̬�����̬ʱ������������ʹ���˲�λ�ֲ��䶳��������

����Ŀ����NaΪ�����ӵ�������ֵ����֪��Ӧ��
��CH4 (g) +2O2 (g) = CO2 (g) +2H2O(l) ��H1 =a kJ mol -1
��CH4(g) + 2O2 (g) = CO2 (g) +2H2O(g) ��H2 =bkJ mol-1
���ܶ��壺�ڱ�״���£���1 mol��̬����AB(g)����Ϊ��̬ԭ��
A(g)��B(g)�������������֪1 mol�����ļ���ΪxkJ�������������� ����ʾ������˵����ȷ����
��ѧ�� | C��O | C��H | O��H |
����/(kJ mol-1) | 798 | 413 | 463 |
A.������x=![]()
B.H2O(g) = H2O(1)����S<0����H=(a �� b) kJ mol -1
C.����4NA��O��H������ʱ����Ӧ�ų�������Ϊa kJ
D.���÷�Ӧ����Ƶ�ԭ��ص�⾫��ͭ�����������0.2NA������ʱ. �����ϵ��۵�������������6.4 g