��Ŀ����
����Ŀ��Ϊ�˼�����84������Һ����Ʒ�ĸ�ʴ�����һ�и���ʦ������������ʹ�õ��Ƕ�����������Ƭ����������(ClO2)��һ�ֻ���ɫ�д̼�����ζ������ �����۵�Ϊ -59�����е�Ϊ11.0����������ˮ����Ŀǰ�����Ϲ��ϵ���������Ч�����ס���ȫ��ɱ�������ʼ�����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ����������(Cl2)���(ClO2)�������и�������ɱ�����������Ҳ��������������DZ��Σ�����л��ȴ��
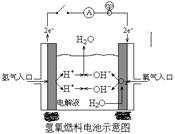
(1)�ҹ�����ɹ����Ƴ���ȡClO2���·������䷴Ӧ���۹�����ͼ��ʾ��
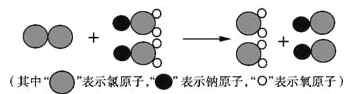
�÷�Ӧ�Ļ�ѧ����ʽΪ ____________��
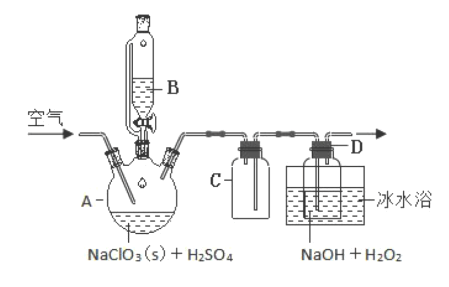
��.ClO2������������������ ��Ũ�ȹ���ʱ�����ֽ⣬Ϊ���������������������Ƴ�NaClO2���� ��ģ�ҵ���ù������ⷨ�Ʊ�NaClO2�����ʵ��װ����ͼ��ʾ��
��֪��H2O2�е� 150����A�еĻ�ѧ����ʽ��2NaClO3+H2O2+H2SO4=2ClO2��+O2��+Na2SO4+2H2O
(2)NaClO3�������� A�У�����B�е�ҩƷ��________(д��ѧʽ)��
(3)�������B �ijɷ�Һ©����ʵ������п��ܻ���ֵ�ʵ������_______��
(4)�� A װ���ͨ��������������Ǹϳ�ClO2Ȼ��ͨ��C �ٵ� D �з�Ӧ��ͨ�������ܹ���ģ��������ٹ���ʱClO2���ܱ�������գ�ͨ����Ҳ���ܹ�������ԭ����_________��
(5)��ˮԡ��ȴ��Ŀ����_____��
a.����NaClO2���ܽ�� b.����H2O2�ķֽ�
c.ʹClO2��ΪҺ̬ d. �ӿ췴Ӧ����
��.���ȶ��Զ���������Һ���ǵ���ɫ��Һ�壬�㷺Ӧ����ʳƷ�����������ɱ��������ClO2�ȶ��Խϲ���ȶ��Զ���������Һ������̼����Ϊ�ȶ�������Ч�ɷ�ΪNaClO2��
ij����ѧϰС���ͬѧ��֤ʵ���е���Ч�ɷֲ��ⶨ�������ȵĺ���(����Ʒ���ᷴӦ�����������ȵ���������Ʒ�����ı�ֵ������)����ش��������⣺
(6)Ϊ֤ʵ���ȶ��Զ���������Һ���к��������ӣ������ǣ�______��
(7)Ϊ�ⶨ���ȶ��Զ���������Һ���ж������ȵĺ������ֽ������²�������ȡ mg(2g ����)������������ƿ�У����Һ©���м���10mL������Һ ��������ƿ�м���4g �⻯�أ��� 100 mLˮ�ܽ�� ���ټ� 3 mL ������Һ ���� �ڲ���Һ����м���ˮ��������Һ©���е�������Һ������ƿ�У��ر�����������������ƿ��ʹ�����Ķ�����������ȫ��ͨ����������ƿ�б����գ���������Һ����е�ˮ��Һ������ƿ�У����뼸�ε�����Һ����cmo l/L��������Ʊ���Һ�ζ�����ɫ��ʧ (![]() )�� ����ȥVmL �����������Һ��
)�� ����ȥVmL �����������Һ��
��NaClO2�����ᷴӦ����ClO2(��ԭ����ΪCl-)�÷�Ӧ�Ļ�ѧ����ʽΪ��_______��
��ClO2ͨ����ƿ�����Ե⻯����Һ��Ӧ���������ȱ���ԭΪ�����ӣ��÷�Ӧ�����ӷ���ʽΪ��_______��
�ۡ��ȶ��Զ���������Һ���У�ClO2����������Ϊ_____(��m��c��V��ʾ)��
���𰸡�2NaClO2+Cl2=2NaCl+2ClO2 H2O2 Һ����˳������ �������ٹ���ʱ��ClO2���ܼ�ʱ�����ߣ�Ũ�ȹ��ߵ��·ֽ� abc ��ɫ��Ӧ����ɫ���� 5NaClO2+4HCl=4ClO2+5NaCl+2H2O 2ClO2+10I-+8H+=2C1-+5I2+4H2O ![]() %
%
��������
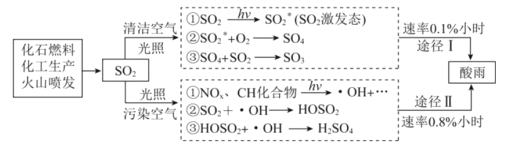
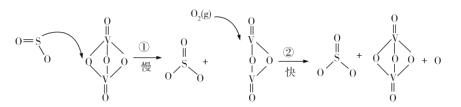
�����۽ṹ�����ó���Ӧ�Ļ�ѧ����ʽ�����÷�Ӧ2NaClO3+ H2O2+ H2SO4= 2ClO2��+ O2�� +Na2SO4+2H2O�Ƶ�ClO2�����ÿ��������ɵ�ClO2�������������ơ�˫��ˮ��Ӧ����NaClO2�����ñ�ˮԡ��ClO2��ΪҺ�壬����˫��ˮ�ֽ⣬��������NaClO2����NaClO2�����ᷴӦ����ClO2��ClO2��KI��Ӧ���ɵ��ʵ⣬������ָʾ������������ƽ��еζ���
(1)�����۽ṹ������Ӧ�Ļ�ѧ����ʽΪ2NaClO2+Cl2=2NaCl+2ClO2��
(2)����A�з�Ӧ��ó�����B�е�ҩƷ��H2O2��
(3)�������B �ijɷ�Һ©������Һ©����������ƿ�е�ѹǿ����ͬ�����ʵ������п��ܻ���ֵ�ʵ������Һ����˳�����䣻
(4)�� A װ���ͨ��������������Ǹϳ�ClO2Ȼ��ͨ��C �ٵ� D �з�Ӧ��ͨ�������ܹ���ģ��������ٹ���ʱClO2���ܱ�������գ�ͨ����Ҳ���ܹ���������������ϢClO2������������������ ��Ũ�ȹ���ʱ�����ֽ⣬��˿������ٹ���ʱ��ClO2���ܼ�ʱ�����ߣ�Ũ�ȹ��ߵ��·ֽ⣻
(5)����������Ϣ��������(ClO2)�е�Ϊ11.0����������ˮ����˱�ˮԡ��ȴ��Ŀ����ʹClO2��ΪҺ̬������ֵķ�Ӧ���ɵ�NaClO2������H2O2�ķֽ⣬��������ʣ�����NaClO2���ܽ�ȣ�ʹ�ĸ���������NaClO2���ʴ�Ϊabc��
(6)Ϊ֤ʵ���ȶ��Զ���������Һ���к��������ӣ������ǣ���ɫ��Ӧ����ɫ���飻
(7)��NaClO2�����ᷴӦ����ClO2��NaCl��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��5NaClO2+4HCl=4ClO2+5NaCl+2H2O��
��ClO2ͨ����ƿ�����Ե⻯����Һ��Ӧ���������ȱ���ԭΪ�����ӣ������ӱ�Ϊ���ʵ⣬��Ӧ�����ӷ���ʽΪ��2ClO2+10I-+8H+=2C1-+5I2+4H2O��
�ۡ��ȶ��Զ���������Һ���У�����ClO2��5![]() ��n(ClO2) =
��n(ClO2) =![]() ��c molL1��V��103L =
��c molL1��V��103L =![]() mol��ClO2����������Ϊ
mol��ClO2����������Ϊ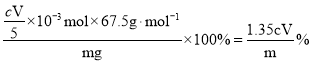 ��
��

 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д�����Ŀ����������ȥ�����ʵķ����У�����ʵ��Ŀ�ĵ���
����(����) | ���� | |
A | SO2(H2S) | ͨ�����Ը��������Һ |
B | Cl2(HCl) | ͨ�����͵�ʳ��ˮ |
C | N2(O2) | ͨ�����ȵ�ͭ˿�� |
D | NO(NO2) | ͨ������������Һ |
A.AB.BC.CD.D