��Ŀ����
����Ŀ����ͼ��ʾ������ʵ��װ���У���Һ�������Ϊ200mL����ʼʱ�������Һ��Ũ�Ⱦ�Ϊ0.1mol/L������һ��ʱ���õ����Ͼ�ͨ��0.02mol���ӣ�����������Һ����ı仯����������������ȷ���ǣ� ��
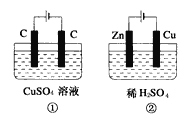
A. ����������������1��>��2��
B. ��ҺPH�ı仯����1������2����С
C. �缫���������ʵ���������1��>��2��
D. �缫��Ӧʽ����1����������40H--4e-=2H2O+O2����2���и�����2H++2e-=H2��
���𰸡�C
����������1������ӵ�Դ���ǵ�������2��û����ӵ�Դ����ԭ��أ�п�Ļ����Դ���ͭ�ģ�����п�Ǹ�����ͭ�����������������ͨ��0.02mol���ӣ���A����1����������4OH--4e-=2H2O+O2��������0.005molO2����2��������ӦΪ2H++2e-=H2��������0.01molH2�����������������1������2����A������B����1������OH-������H+����ҺpH��С����2������H+����ҺpH����B����C����1���������൱��CuO����������=0.02mol/2��80g/mol=0.8g����2����������������������=0.02mol/2g/mol��2=0.02g�����ԣ�1������2����C��ȷ��D����2���и�����ӦΪZn-2e-=Zn2+��D��������ѡC��