��Ŀ����
5����������Ӱ�����ǵ������뽡����ij�����������п��ܺ������¿����������ӣ�Na+��NH4+��Mg2+��Al3+��SO42-��NO3-��Cl-��ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ�������������Һ����Ʋ���������µ�ʵ�飺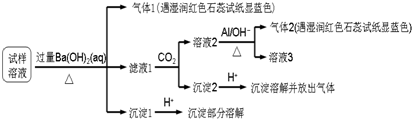
��֪��3NO3-+8Al+5OH-+2H2O$\stackrel{��}{��}$3NH3+8AlO2-�������ϵ�ʵ�����������ͬѧ�ó��Ľ��۲���ȷ���ǣ�������
| A�� | �����п϶�����NH4+��Mg2+��SO42-��NO3- | |
| B�� | �����п��ܺ���Al3+ | |
| C�� | �������п��ܴ���NaNO3��NH4Cl��MgSO4 | |
| D�� | �����в����ܴ���Na+��Cl- |
���� ������Һ�м������Ba��OH��2�����ȣ����ɵ�����1��������1������NH3���������к���NH4+������Һ��ͨ��CO2���õ���Һ2������2����Һ2�м���Al��NO3-+A1+OH-+H2O��NH3��+[Al��OH��4]-����������2����������NH3��������֪����֪����Һ2�к���NO3-������Ԫ���غ�֪��ԭ��Һ�к���NO3-����Һ1��ͨ��CO2���õ�����2�������2�м����ᣬ�����ܽⲢ�ų����壬˵������2��̼�ᱵ��������̼���Σ�����1��������������ܽ⣬���ᱵ�������ᣬ˵��ԭ����Һ�к���SO42-���ܺ���Ba��OH��2��Ӧ������������ij�������������֪���ó���ΪMg��OH��2��������Һ�к���Mg 2+��������ѡ��������
��� �⣺������Һ�м������Ba��OH��2�����ȣ����ɵ�����1��������1������NH3���������к���NH4+������Һ��ͨ��CO2���õ���Һ2������2����Һ2�м���Al��NO3-+A1+OH-+H2O��NH3��+[Al��OH��4]-����������2����������NH3��������֪����֪����Һ2�к���NO3-������Ԫ���غ�֪��ԭ��Һ�к���NO3-����Һ1��ͨ��CO2���õ�����2�������2�м����ᣬ�����ܽⲢ�ų����壬˵������2��̼�ᱵ��������̼���Σ�����1��������������ܽ⣬���ᱵ�������ᣬ˵��ԭ����Һ�к���SO42-���ܺ���Ba��OH��2��Ӧ������������ij�������������֪���ó���ΪMg��OH��2��������Һ�к���Mg 2+��
A���������Ϸ�����֪��Һ�к���NH4+��Mg2+��SO42-��NO3-����A��ȷ��
B������ʵ���������ȷ���Ƿ���Al3+���������п��ܺ���Al3+����B��ȷ��
C���������Ϸ�����֪��Һ�к���NH4+��Mg2+��SO42-��NO3-����������п��ܴ���NaNO3��NH4Cl��MgSO4����C��ȷ��
D������ʵ����ȷ���Ƿ���Na+��Cl-��������Һ�п��ܺ���Na+��Cl-����D����
��ѡD��
���� ���⿼�������ʵ��ƶϣ���ȷ���ʵ����ʼ����ⷴӦ�����ǽⱾ��ؼ����������ʵ��ܽ��ԡ����ʵ����ʼ������Ϣ�����������Ŀ�Ѷ��еȣ�

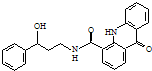
| A�� | ��X�ķ���ʽΪC23H25N2O3 | |
| B�� | ÿ��X�����к���2������̼ԭ�� | |
| C�� | 1 mol X�������9 mol H2�����ӳɷ�Ӧ | |
| D�� | X�ܷ���ˮ�⡢��������ȥ��Ӧ |
| A�� | ��λ���ʵ�����������ռ�������������Ħ����� | |
| B�� | Ħ�������ʵ����ĵ�λ | |
| C�� | �����ӵ���������6.02��1023 | |
| D�� | CO2��Ħ������Ϊ44g |
| A�� | Cu | B�� | Fe | C�� | SO2 | D�� | I- |
| �� | �� | �� | ||
| ��ʼ���ʵ��� | n��SO2��/mol | 0.4 | 0.4 | 0.8 |
| n��O2��/mol | 0.24 | 0.48 | 0.48 | |
| SO2��ƽ��ת���� | 80% | ��1 | ��2 | |
| A�� | ���з�Ӧ��ƽ�ⳣ�������� | B�� | ƽ��ʱ��SO2��ת���ʣ���1��80%����2 | ||
| C�� | ���¶��£�����ƽ�ⳣ��ֵΪ400 | D�� | ƽ��ʱ������c��SO3���Ǽ��е�2�� |
 Ϊ��̽����������Է�Ӧ2NO2��g��?N2O4��g����H=-57kJ•mol-1��Ӱ�죬�������������������䣬���¶�ΪT1��T2ʱ��ͨ��ʵ��õ�ƽ����ϵ��NO2�����������ѹǿ�仯���ߣ�ʵ������ͼ��ʾ������˵����ȷ���ǣ�������
Ϊ��̽����������Է�Ӧ2NO2��g��?N2O4��g����H=-57kJ•mol-1��Ӱ�죬�������������������䣬���¶�ΪT1��T2ʱ��ͨ��ʵ��õ�ƽ����ϵ��NO2�����������ѹǿ�仯���ߣ�ʵ������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | a��c���������ƽ����Է���������a��c | |
| B�� | a��c�����������ɫ��adz��c�� | |
| C�� | b��c�����ƽ�ⳣ����Kb=Kc | |
| D�� | ״̬aͨ�������¶ȿɱ��״̬b |
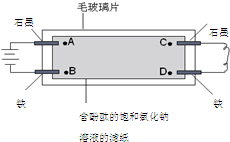 ��ͼ��ʾ����ë����Ƭ�Ϸ���һ�Ž��к���̪�ı����Ȼ�����Һ����ֽ����ֽ�ϵ���A��B��C��D�ĸ��㣨ָ�����缫����Һ�����й�˵����ȷ���ǣ�������
��ͼ��ʾ����ë����Ƭ�Ϸ���һ�Ž��к���̪�ı����Ȼ�����Һ����ֽ����ֽ�ϵ���A��B��C��D�ĸ��㣨ָ�����缫����Һ�����й�˵����ȷ���ǣ�������| A�� | A���B���ȳ��ֺ�ɫ | B�� | B���A���ȳ��ֺ�ɫ | ||
| C�� | C���A���ȳ��ֺ�ɫ | D�� | D���C���ȳ��ֺ�ɫ |
| ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
| A | ����CH3CH2Br��NaOH��Һ�Ƿ�����Ӧ | ��CH3CH2Br��NaOH��Һ���ȣ���ȴ��ȡ�ϲ���Һ����AgNO3��Һ���۲��Ƿ��������ɫ���� |
| B | ����ĥ����AlƬͶ��һ��Ũ�ȵ�CuCl2��Һ���������ݲ��й������ɣ����ˣ�������м�������İ�ˮ�����岿���ܽ� | Al��CuCl2��Һ��Ӧ����H2��Cu��OH��2���� |
| C | ����Fe��NO3��2�����Ƿ����������� | ��Fe��NO3��2��Ʒ����ϡH2SO4�μ�KSCN��Һ���۲���Һ�Ƿ��ɺ�ɫ |
| D | �����Ҵ���Ũ���Ṳ���Ƿ������ϩ | ���Ҵ���Ũ���Ṳ����170�棬��������ͨ�����Ը��������Һ�У��۲���Һ�Ƿ���ɫ |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ����
����
 ��
�� ��
�� ��
�� ��
�� ��
�� ��
��