题目内容
【题目】东晋《华阳国志·南中志》卷四中已有关于白铜的记载,云南镍白铜(铜镍合金)闻名中外,曾主要用于造币,亦可用于制作仿银饰品。回答下列问题:
(1)镍元素基态原子的电子排布式为______,3d能级上的未成对电子数为_______。
(2)硫酸镍溶于氨水形成[Ni(NH3)6]SO4蓝色溶液。
①[Ni(NH3)6]SO4中阴离子的立体构型是________。
②在[Ni(NH3)6]SO4中Ni2+与NH3之间形成的化学键称为_______,提供孤电子对的成键原子是________。
(3)单质铜及镍都是由______键形成的晶体;元素铜与镍的第二电离能分别为:ICu=1 958 kJ·mol–1、INi=1 753 kJ·mol–1,ICu> INi的原因是_______________________。
【答案】1s22s22p63s23p63d84s2或 [Ar]3d84s2 2 正四面体 配位键 N 金属 铜的第二电离能失去的是全充满的3d10电子,镍失去的是4s1电子
【解析】
(1)Ni元素原子核外电子数为28,结合能量最低原理书写核外电子排布式;
(2)①根据价层电子对个数=σ键个数+孤电子对个数分析判断SO42-的空间构型;②Ni2+为中心原子,NH3为配体,据此解答;
(3)单质铜及镍都属于金属晶体;Cu+的外围电子排布为3d10,Ni+的外围电子排布为3d84s1,据此分析解答。
(1)Ni元素原子核外电子数为28,核外电子排布式为:1s22s22p63s23p63d84s2,3d能级上的未成对电子数为2,故答案为:1s22s22p63s23p63d84s2;2;
(2)①[Ni(NH3)6]SO4中阴离子为SO42-,SO42-中S原子的孤电子对数=![]() =0,价层电子对数=4+0=4,离子空间构型为正四面体,故答案为:正四面体;
=0,价层电子对数=4+0=4,离子空间构型为正四面体,故答案为:正四面体;
②在[Ni(NH3)6]SO4中Ni2+提供空轨道,NH3中N原子含有孤电子对,二者之间形成配位键,故答案为:配位键;N;
(3)单质铜及镍都属于金属晶体,都是由金属键形成的晶体;Cu+的外围电子排布为3d10,呈全充满状态,比较稳定,Ni+的外围电子排布为3d84s1,Cu+的核外电子排布更稳定,失去第二个电子更难,因此铜的第二电离能高于镍的第二电离能,故答案为:金属;铜的第二电离能失去的是全充满的3d10电子,镍失去的是4s1电子。

 每日10分钟口算心算速算天天练系列答案
每日10分钟口算心算速算天天练系列答案【题目】三氯乙醛(CCl3CHO)是生产农药、医药的重要中间体,实验室制备三氯乙醛的反应装置示意图(加热装置未画出)和有关数据如下:
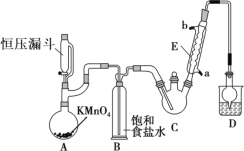
①制备反应原理:C2H5OH+4Cl2→CCl3CHO+5HCl
②相关物质的相对分子质量及部分物理性质:
相对分子质量 | 熔点/℃ | 沸点/℃ | 溶解性 | |
C2H5OH | 46 | -114.1 | 78.3 | 与水互溶 |
CCl3CHO | 147.5 | -57.5 | 97.8 | 可溶于水、乙醇 |
CCl3COOH | 163.5 | 58 | 198 | 可溶于水、乙醇、三氯乙醛 |
C2H5Cl | 64.5 | -138.7 | 12.3 | 微溶于水,可溶于乙醇 |
(1)恒压漏斗中盛放的试剂的名称是_____,盛放KMnO4仪器的名称是_____。
(2)反应过程中C2H5OH和HCl可能会生成副产物C2H5Cl,同时CCl3CHO(三氯乙醛)也能被次氯酸继续氧化生成CCl3COOH(三氯乙酸),写出三氯乙醛被次氯酸氧化生成三氯乙酸的化学方程式:_____。
(3)该设计流程中存在一处缺陷是_____,导致引起的后果是_____,装置B的作用是______。
(4)反应结束后,有人提出先将C中的混合物冷却到室温,再用分液的方法分离出三氯乙酸。你认为此方案是否可行_____(填是或否),原因是_____。
(5)测定产品纯度:称取产品0.36g配成待测溶液,加入0.1000molL1碘标准溶液20.00mL,再加入适量Na2CO3溶液,反应完全后,加盐酸调节溶液的pH,立即用0.02000molL1Na2S2O3溶液滴定至终点。进行三次平行实验,测得平均消耗Na2S2O3溶液20.00mL。则产品的纯度为_____(计算结果保留四位有效数字)。滴定原理:CCl3CHO+OH-=CHCl3+HCOO-、HCOO-+I2=H++2I-+CO2、I2+2S2O32-=2I-+S4O62-
【题目】探究电场作用下阴阳离子的迁移。a、b、c、d 均为石墨电极,电极间距4cm。将pH试纸用不同浓度Na2SO4溶液充分润湿,进行如下实验:
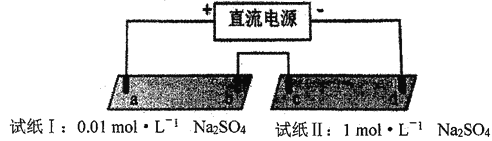
实验现象:
时间 | 试纸I | 试纸II |
lmin | a极附近试纸变红,b极附近试纸变蓝 | c极附近试纸变红,d极附近…… |
10min | 红色区和蓝色区不断向中间扩展,相遇时红色区约2.7cm,蓝色区约1.3cm | 两极颜色范围扩大不明显,试纸大部分仍为黄色 |
下列说法不正确的是
A. d极附近试纸变蓝
B. a极附近试纸变红的原因是:2H2O+2e-= H2↑+2OH-
C. 对比试纸I和试纸II的现象,说明电解质浓度环境影响H+和OH-的迁移
D. 试纸I的现象说明,此环境中H+的迁移速率比OH-快