��Ŀ����
����Ŀ��![]() ������
������![]() ��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ�
��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ�

�ش��������⣺
(1)��ʵ��װ���Ͽ���ͼ����ȱ��һ�ֲ������������ֲ���������������____________________���ܷ����ͭ˿_______(�����ܡ�����)��ԭ����____________________________
(2)���ձ����粻��Ӳֽ�壬��õ��к�����ֵ��__________(����ƫ������ƫС��������Ӱ����)��
(3)ʵ���и���![]() �����
�����![]() ��Һ���з�Ӧ��������ʵ����ȣ����ų�������__________(����ƫ���������������ƫС��)�������к���__________(��������������������)��
��Һ���з�Ӧ��������ʵ����ȣ����ų�������__________(����ƫ���������������ƫС��)�������к���__________(��������������������)��
(4)����ͬŨ�Ⱥ�����İ�ˮ����![]() ��Һ��������ʵ�飬��õ��к�����ֵ��__________(����ƫ������ƫС��������Ӱ����)��
��Һ��������ʵ�飬��õ��к�����ֵ��__________(����ƫ������ƫС��������Ӱ����)��
(5)ȡ![]() ��Һ��
��Һ��![]() ������Һ����ʵ�飬ʵ���������±���
������Һ����ʵ�飬ʵ���������±���
�¶� ʵ����� | ��ʼ�¶� | ��ֹ�¶�
| �¶Ȳ�ƽ��ֵ
| ||
|
| ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | _________________ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
���к���![]() __________(ȡС�����һλ��)(������
__________(ȡС�����һλ��)(������![]() ��Һ��������Һ���ܶȾ�ȡ
��Һ��������Һ���ܶȾ�ȡ![]() ���кͺ�������Һ�ı�����ȡ
���кͺ�������Һ�ı�����ȡ![]() )
)
���𰸡����β�������� �� ��Ϊͭ���ȿ죬������ʧ�� ƫС ��� ��� ƫС 4.0�� ![]()
��������
�к��ȵIJⶨʵ�飬�к��ȵ���ֵҪȷ������������ʧ����˺ܶ��������Ϊ�˱�֤��������ʧ���к��ȵļ��㹫ʽΪ![]() ���к��ȵ���ֵΪ��H�ľ���ֵ�������к���һ����ǿ�ᡢǿ���ϡ��Һ�ⶨ�������ǿ�ᡢǿ���Ũ��Һ����Ӧ��Һ���ϡ��ʱҪ���ȣ���ֵ��ƫ����������ᡢ�����ӦʱҪ���룬������ʵ���Ҫ���ȣ���ֵƫС��
���к��ȵ���ֵΪ��H�ľ���ֵ�������к���һ����ǿ�ᡢǿ���ϡ��Һ�ⶨ�������ǿ�ᡢǿ���Ũ��Һ����Ӧ��Һ���ϡ��ʱҪ���ȣ���ֵ��ƫ����������ᡢ�����ӦʱҪ���룬������ʵ���Ҫ���ȣ���ֵƫС��
(1)��װ���п��Կ���������ʹ�����Һ��ֻ�ϵ�������Ϊ���β�������������Dz���ʹ�ý�����������Ϊ���������ĵ����ԱȲ����ã��������������ʧ�ϴ�Ϊ���β��������������Ϊͭ���ȿ죬������ʧ��
(2) ���ձ����粻��Ӳֽ�壬��Ӧ����Һֱ�����������Ӵ����Ƚ������ӳ�֣�������ʧ��õ�����������С�����ù�ʽ![]() ��QƫС����Hƫ��ע���HΪ��ֵ����ˡ�H�ľ���ֵƫС�����к��ȵ���ֵƫС����ΪƫС��
��QƫС����Hƫ��ע���HΪ��ֵ����ˡ�H�ľ���ֵƫС�����к��ȵ���ֵƫС����ΪƫС��
(3)�����60mL 0.5mol��L��1�������50mL 0.50mol��L��1��NaOH��Һʵ�飬NaOH��ȫ��Ӧ��������![]() ��ˮ��ԭʵ��ķ�Ӧ������ȫ��Ӧ����Ҳ������0.025mol��ˮ���ų�����������ȵġ��к���Ϊ��ϡ��Һ������к�����1molˮ�ķ�Ӧ�ȣ����������Ƕ��٣�������к��ȵ���ֵʱ����Ҫ���������1molˮ�ų��������������к��ȵ���ֵҲ��ȡ���Ϊ��ȣ� ��ȣ�
��ˮ��ԭʵ��ķ�Ӧ������ȫ��Ӧ����Ҳ������0.025mol��ˮ���ų�����������ȵġ��к���Ϊ��ϡ��Һ������к�����1molˮ�ķ�Ӧ�ȣ����������Ƕ��٣�������к��ȵ���ֵʱ����Ҫ���������1molˮ�ų��������������к��ȵ���ֵҲ��ȡ���Ϊ��ȣ� ��ȣ�
(4) ����������������ǿ��ǿ�Ӧ����ӦʱҪ���룬������ʵ���Ҫ���ȣ��к�����ֵ��ƫС����ΪƫС��
(5)���¶ȵ�ƽ��ֵ�����Ȱ�ÿ��ʵ����²��������ʵ��1���²�Ϊ30.1-26.1=4�棬ͬ��ʵ��2���²�Ϊ6.1�棬ʵ��3���²�Ϊ3.9�棬ʵ��4���²�Ϊ4.1�棬ʵ��2���¶Ȳ�����������ʵ���������Ҫ�������²��ƽ��ֵΪ![]() �����ù�ʽ
�����ù�ʽ![]() ��������c=4.2J/(g����)��
��������c=4.2J/(g����)��![]() ��t2- t1=4�档NaOH�����ʵ���Ϊ0.025mol��H2SO4�����ʵ���Ϊ0.06mol��NaOH��ȫ��Ӧ�꣬nˮ=0.025mol�����빫ʽ
��t2- t1=4�档NaOH�����ʵ���Ϊ0.025mol��H2SO4�����ʵ���Ϊ0.06mol��NaOH��ȫ��Ӧ�꣬nˮ=0.025mol�����빫ʽ![]() ����Ϊ4.0�� -53.8kJ/mol��
����Ϊ4.0�� -53.8kJ/mol��

����Ŀ��N2O5��һ��������������һ���¶��·�����Ӧ2N2O5(g)4NO2(g)��O2(g)����H>0, T1�¶��µIJ���ʵ���������±���ʾ��
t/s | 0 | 500 | 1 000 | 1 500 |
c(N2O5)/(mol/L) | 5.00 | 3.52 | 2.50 | 2.50 |
����˵����ȷ����
A. �÷�Ӧ���κ��¶��¾����Է�����
B. T1�¶��µ�ƽ�ⳣ��ΪK1��125��1 000 sʱN2O5(g)ת����Ϊ50%
C. ������������ʱ��T2�¶��·�Ӧ��1 000 sʱ���N2O5(g)Ũ��Ϊ2.98 mol/L����T1<T2
D. T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2����T1>T2����K1<K2
����Ŀ����ѧС���������������������װ��(��ͼ)���û������Ʊ�����ϩ��
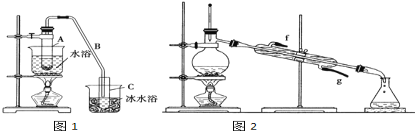
��֪��
��Է������� | �ܶ�(g/cm3) | �۵�(��) | �е�(��) | �ܽ��� | |
������ | 100 | 0.96 | 25 | 161 | ������ˮ |
����ϩ | 82 | 0.81 | ��103 | 83 | ������ˮ |
��1���Ʊ���Ʒ��
��12.5mL�����������Թ�A��,�ټ���1mLŨ����,ҡ�Ⱥ�������Ƭ(��ֹ����)��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
������B���˵�������е�������_________.
���Թ�C���ڱ�ˮԡ�е�Ŀ����____________________________.
��2���Ʊ���Ʒ��
������ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ,����ֹ���ֲ�,����ϩ��______��(����������������),��Һ����_______(��ѡ����ĸ)ϴ�ӡ�
a��KMnO4��Һ�� ��b��ϡH2SO4�� ��c��Na2CO3��Һ
���ٽ�����ϩ����ͼ2װ����������ʱҪ������ʯ�ң�Ŀ����__________________________��
�������ռ���Ʒʱ,ʵ���ƵõĻ���ϩ��Ʒ�����������۲���,���ܵ�ԭ����_______(��ѡ����ĸ).
a. ����ʱ��70����ʼ�ռ���Ʒ b. ������ʵ����������
c. �Ʊ���Ʒʱ���������Ʒһ��������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_______(��ѡ����ĸ)��
a. �ֱ�����ý����� b. �ֱ�������Ը��������Һ
c. �ֱ�ⶨ�е� d. �ֱ����FeCl3��Һ
��4��������յõ���������ϩ��Ʒ����Ϊ8.2 g, ���ʵ�����õ��Ļ���ϩ������___________������3λ��Ч���֣���


