��Ŀ����
(11��) ��ͬ�����ܡ����Ƚ������Թ��ɸ��ºϽ𡢵��ȺϽ𡢾��ܺϽ�ȣ����ں��ա�����������DZ��ȹ�ҵ���š�
(1)��ԭ�ӵĻ�̬�����Ų�ʽΪ_____________________ _____________��
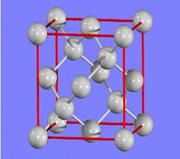
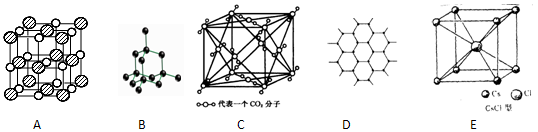
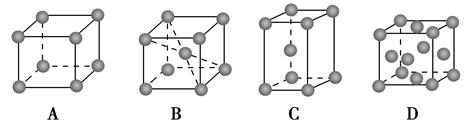
(2)�������Ķѻ���ʽ����A2�ͣ��侧��ʾ��ͼΪ________(�����)��

(3)�Ȼ�����(CrO2Cl2)������Ϊ����ɫҺ�壬�۵㣭96.5 �棬�е�117 �棬�����ͪ(CH3COCH3)��CCl4��CS2�Ȼ��ܡ�
�ٹ�̬CrO2Cl2����______ __���壻
�ڱ�ͪ��̼ԭ�Ӳ�ȡ���ӻ���ʽΪ_ __��
��CS2����____ _ ___(����ԡ��Ǽ��ԡ�)���ӡ�
(4)��3�۸��������K[Cr(C2O4)2(H2O)2]�У�������________________����C2O42����Ϊ�ȵ�����ķ�����(�ѧʽ)___ _____��
(5)CrCl3��6H2O(��Է�������Ϊ266.5)�����ֲ�ͬ��ɫ���칹�壺[Cr(H2O)6]Cl3��[Cr(H2O)5Cl]Cl2��H2O��[Cr(H2O)4Cl2]Cl��2H2O��Ϊ�ⶨ����CrCl3��Һ�����İ���ɫ�����������칹�壬ȡ2.665 g CrCl3��6H2O�����Һ���μ�����AgNO3��Һ���õ�����1.435 g�����칹��Ϊ_________ ___________(�ѧʽ)��
(1)��ԭ�ӵĻ�̬�����Ų�ʽΪ_____________________ _____________��
(2)�������Ķѻ���ʽ����A2�ͣ��侧��ʾ��ͼΪ________(�����)��


(3)�Ȼ�����(CrO2Cl2)������Ϊ����ɫҺ�壬�۵㣭96.5 �棬�е�117 �棬�����ͪ(CH3COCH3)��CCl4��CS2�Ȼ��ܡ�
�ٹ�̬CrO2Cl2����______ __���壻
�ڱ�ͪ��̼ԭ�Ӳ�ȡ���ӻ���ʽΪ_ __��
��CS2����____ _ ___(����ԡ��Ǽ��ԡ�)���ӡ�
(4)��3�۸��������K[Cr(C2O4)2(H2O)2]�У�������________________����C2O42����Ϊ�ȵ�����ķ�����(�ѧʽ)___ _____��
(5)CrCl3��6H2O(��Է�������Ϊ266.5)�����ֲ�ͬ��ɫ���칹�壺[Cr(H2O)6]Cl3��[Cr(H2O)5Cl]Cl2��H2O��[Cr(H2O)4Cl2]Cl��2H2O��Ϊ�ⶨ����CrCl3��Һ�����İ���ɫ�����������칹�壬ȡ2.665 g CrCl3��6H2O�����Һ���μ�����AgNO3��Һ���õ�����1.435 g�����칹��Ϊ_________ ___________(�ѧʽ)��
(1)1s22s22p63s23p63d74s2��[Ar]3d74s2��2�֣�(2)B��1�֣�(3)�ٷ��ӡ���1�֣���sp2�ӻ���sp3�ӻ�������1�֣��۷Ǽ��ԡ���1�֣�
(4)C2O42����H2O��N2O4 ����1�֣�(5)[Cr(H2O)4Cl2]Cl��2H2O��1�֣�
(4)C2O42����H2O��N2O4 ����1�֣�(5)[Cr(H2O)4Cl2]Cl��2H2O��1�֣�
��1�����ݹ���ԭ�����γ���ԭ�ӵĻ�̬�����Ų�ʽ��
��2���������Ķѻ���ʽ����A2�ͣ�����ѡ��AD����ȷ��ѡ��C���������壬��������ѡ��B��ȷ��
��3����ͪ(CH3COCH3)��CCl4��CS2�Ⱦ����ڷǼ��Է��ӣ�������������ԭ�����жϣ���̬CrO2Cl2���ڷ��Ӿ��塣���ݱ�ͪ�Ľṹ��ʽ��֪��2�����ϵ�̼ԭ����sp3�ӻ����ʻ�̼ԭ����sp2�ӻ���CS2�е�̼ԭ����sp�ӻ�������ֱ���ͽṹ���ǷǼ��Է��ӡ�
��3���ṩ�¶Ե��ӵ������壬���Ըû�������C2O42����H2O�����塣ԭ�����͵���������ȵ��ǵȵ����壬��˺�C2O42����Ϊ�ȵ�����ķ�����N2O4��
��4���Ȼ���������1.435g�����ʵ�����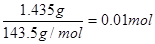 ��2.665 g CrCl3��6H2O�����ʵ�����0.01mol����˵��������ֻ��1�������ӿ��Ե����������2�������壬�����칹����[Cr(H2O)4Cl2]Cl��2H2O��
��2.665 g CrCl3��6H2O�����ʵ�����0.01mol����˵��������ֻ��1�������ӿ��Ե����������2�������壬�����칹����[Cr(H2O)4Cl2]Cl��2H2O��
��2���������Ķѻ���ʽ����A2�ͣ�����ѡ��AD����ȷ��ѡ��C���������壬��������ѡ��B��ȷ��
��3����ͪ(CH3COCH3)��CCl4��CS2�Ⱦ����ڷǼ��Է��ӣ�������������ԭ�����жϣ���̬CrO2Cl2���ڷ��Ӿ��塣���ݱ�ͪ�Ľṹ��ʽ��֪��2�����ϵ�̼ԭ����sp3�ӻ����ʻ�̼ԭ����sp2�ӻ���CS2�е�̼ԭ����sp�ӻ�������ֱ���ͽṹ���ǷǼ��Է��ӡ�
��3���ṩ�¶Ե��ӵ������壬���Ըû�������C2O42����H2O�����塣ԭ�����͵���������ȵ��ǵȵ����壬��˺�C2O42����Ϊ�ȵ�����ķ�����N2O4��
��4���Ȼ���������1.435g�����ʵ�����
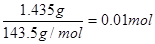 ��2.665 g CrCl3��6H2O�����ʵ�����0.01mol����˵��������ֻ��1�������ӿ��Ե����������2�������壬�����칹����[Cr(H2O)4Cl2]Cl��2H2O��
��2.665 g CrCl3��6H2O�����ʵ�����0.01mol����˵��������ֻ��1�������ӿ��Ե����������2�������壬�����칹����[Cr(H2O)4Cl2]Cl��2H2O��
��ϰ��ϵ�д�
�����Ŀ
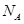 ��ʾ�����ӵ����������������������Ϊ cm3��
��ʾ�����ӵ����������������������Ϊ cm3��