��Ŀ����
VA��Ԫ�ض����γ�RH3�͵��⻯�����NH3��VI���е�H2O�кܶ�����ƣ����ƶϰ������ʣ�
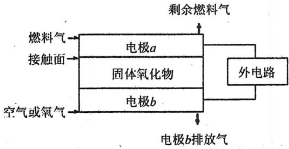
��1��Һ����һ�����õ��ܼ����������Һ����_____���루���ܻ��ܣ���
��2����������350��Cʱ�백������Ӧ�Ļ�ѧ����ʽ��___________________________��
��3��������Ϊ���ľ�Ǽ������������Ļ�����Щ�������Ҫ��Һ���ĺ����������Ϊ�������Һ���൱�ڵ����ϵ�ˮ������������������̼��Ϊ�ǼܵĻ�����ô���������������а������൱����_________��������������൱�Ļ�������__________��
�� �� (2) 2Na + 2NH3 =2NaNH2 + H2��(3) �ǣ� ������

��ϰ��ϵ�д�
�����Ŀ

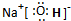

 A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ�Ӱ뾶�� С��BԪ�ص�����������Ӧˮ���������⻯���������Σ�D��Aͬ���壬����Eͬ�� �ڣ�EԪ��ԭ�ӵ��������������������������
A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ�Ӱ뾶�� С��BԪ�ص�����������Ӧˮ���������⻯���������Σ�D��Aͬ���壬����Eͬ�� �ڣ�EԪ��ԭ�ӵ��������������������������