��Ŀ����
12���л���A��������ʵ�������ijͬѧ����AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ��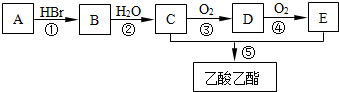
��ش�
��1��E�Ľṹ��ʽ��CH3COOH�������к��еĹ������������Ȼ���
��2����Ӧ�ٵķ�Ӧ�����Ǽӳɷ�Ӧ����Ӧ�١����з�Ӧ���������ͬ���Ǣݣ�����ţ���
��3����A����ֱ��ת��ΪC���䷴Ӧ�Ļ�ѧ����ʽ��CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH��
��4����Ӧ�۵Ļ�ѧ����ʽ��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O����Ӧ�ܵĻ�ѧ����ʽ��2CH3CHO+O2 $\stackrel{����}{��}$2CH3COOH��
��5�����л���A�Ʊ�ʳƷ��װ�����ϵĻ�ѧ����ʽ��

��6��д��������������Ϊͬ���칹������������Ľṹ��ʽCH3CH2CH2COOH����CH3��2CHCOOH��
���� �л���A��������ʵ�������AΪCH2=CH2��A��HBr�����ӳɷ�Ӧ����B��BΪCH3CH2Br��B����ȡ����Ӧ����C��CΪCH3CH2OH��C����������D��DΪCH3CHO��D����������E��EΪCH3COOH�������Ŀ�������
��� �⣺�л���A��������ʵ�������AΪCH2=CH2��A��HBr�����ӳɷ�Ӧ����B��BΪCH3CH2Br��B����ȡ����Ӧ����C��CΪCH3CH2OH��C����������D��DΪCH3CHO��D����������E��EΪCH3COOH��
��1��E�����ᣬ��ṹ��ʽΪCH3COOH���÷����й��������Ȼ����ʴ�Ϊ��CH3COOH���Ȼ���
��2����Ӧ�ٵķ�Ӧ�����Ǽӳɷ�Ӧ������ȡ����Ӧ����Ӧ�١����з�Ӧ���������ͬ���Ǣݣ�
�ʴ�Ϊ���ӳɣ��ݣ�
��3����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����Ӧ����ʽΪCH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH��
�ʴ�Ϊ��CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH��
��4����Ӧ�����Ҵ���������Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O����Ӧ��Ϊ��ȩ��������Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��2CH3CHO+O2 $\stackrel{����}{��}$2CH3COOH��
�ʴ�Ϊ��2CH3CH2OH+O2 $��_{��}^{Cu}$2CH3CHO+2H2O��2CH3CHO+O2 $\stackrel{����}{��}$2CH3COOH��
��5����ϩ�����Ӿ۷�Ӧ���ɾ���ϩ���÷�Ӧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6��������������Ϊͬ���칹������������Ľṹ��ʽ��CH3CH2CH2COOH����CH3��2CHCOOH���ʴ�Ϊ��CH3CH2CH2COOH����CH3��2CHCOOH��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬�漰ϩ��������ȩ�����ᡢ����±����֮���ת������ȷ�����ż������ʡ�������Ӧ���͵�֪ʶ�㼴�ɽ���״�����ͬ���칹���жϣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ���������������Ա���Ի�������Ⱦ | |
| B�� | �Ӵ����е��Ƚ�������ΪԤ�ȷ�Ӧ�����ȴ�������Լ��������Ƶ� | |
| C�� | �������������ŨH2SO4һ��������˽������ɳ�Ʒ�� | |
| D�� | ��ˮ����SO3ʹ֮��Ϊ���� |
| A�� | ��ϩ������8���Ҽ���1���м� | |
| B�� | ��ϩ������3��̼ԭ�Ӷ���sp3�ӻ� | |
| C�� | ��ϩ������ֻ���ڼ��Լ� | |
| D�� | ��ϩ������3��̼ԭ����ͬһֱ���� |
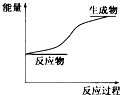
| A�� | ���淴Ӧ������������Ӧ���ʵ����淴Ӧ���� | |
| B�� | �������������ı�ʱ������ѹǿ����Һ�䷴Ӧ������Ӱ�� | |
| C�� | �¶����ߣ�����ʹ���淴Ӧ����Ӧ���ʼӿ죬���淴Ӧ���ʿ��Բ��� | |
| D�� | ʹ�ô�������ʹԭ�����ܷ����ķ�Ӧ��Ϊ���ܷ����� |
| A�� | �������������С�ڷ�Ӧ��������� | |
| B�� | �Ͽ���ѧ�������յ�����С���γɻ�ѧ�����ų������� | |
| C�� | �÷�Ӧ��������ͼ���кͷ�Ӧ | |
| D�� | �÷�Ӧ��������ʽ������ת�����˻�ѧ�� |
| A�� | ����ѹǿ | B�� | �Ӵ�ˮ�� | C�� | �����¶� | D�� | ������� |
 ��ϵͳ������3��4-��������
��ϵͳ������3��4-��������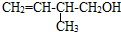 �ļ���ʽ��
�ļ���ʽ��
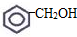 ��
��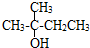 ��
��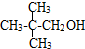 ��
�� ��
��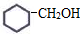
 ��
�� NO���������������Ѹ�ٷ�����Ӧ����ѧ�ҷ������������в��ϵز���NO������ϸ���䴫����Ϣ��NO��������Ѫ��ϵͳ������ϵͳ�Լ��������Χ��ϵͳ�ĵ��أ�
NO���������������Ѹ�ٷ�����Ӧ����ѧ�ҷ������������в��ϵز���NO������ϸ���䴫����Ϣ��NO��������Ѫ��ϵͳ������ϵͳ�Լ��������Χ��ϵͳ�ĵ��أ�