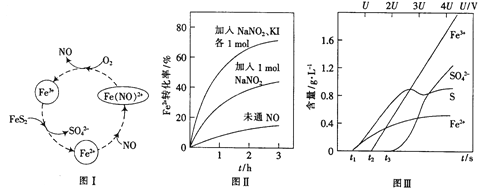
��Ŀ����
����Ŀ��A��B��C��D��EΪԭ��������������Ķ���������Ԫ�أ��ֲ���������ͬ���ڡ�X��Y��Z��WΪ��ЩԪ���γɵĻ����XΪ��Ԫ��������Ϊǿ����ʣ�W��ˮ��Һ�ʼ��ԣ����ʵ�ת����ϵ��ͼ��ʾ������˵������ȷ����

A. ��Ӧ�ļ����Ӱ뾶��C>D>B
B. D��E�γɵĻ�����Ϊ���м��Թ��ۼ��Ĺ��ۻ�����
C. ���C��E�γɵĻ�����ˮ��Һ��������C��E��Ӧ�ĵ���
D. ��A��B��E�γɵĻ����ﶼ���й��ۼ�����Һ����ǿ����
���𰸡�B
��������
����A��B��C��D��EΪԭ��������������Ķ���������Ԫ�أ��ֲ���������ͬ���ڡ�X��Y��Z��WΪ��ЩԪ���γɵĻ����XΪ��Ԫ��������Ϊǿ����ʣ�W��ˮ��Һ�ʼ��Խ��ͼ����֪��DΪAl��X��ҺΪHCl��Y��ҺΪNaOH��Һ��Z��ҺΪAlCl3��W��ҺΪNaAlO2������Ԫ��A��B��C��D��E�ֱ�Ϊ��H��O��Na��Al��Cl�����Ԫ�ص�������ʽ����жϡ�
A. B��C��D��Ӧ��Ԫ��ΪO��Na��Al��������Ӱ뾶��O2->Na+>Al3+,��A����
B. D��E�ֱ�ΪAl��Cl ��D��E�γɵĻ�����ΪAlCl3��Ϊ���м��Թ��ۼ��Ĺ��ۻ������B��ȷ��
C.CΪNa ��EΪCl��C��E�γɵĻ���ΪNaCl���������ˮ��Һ��������H2��Cl2��C����
D. A��B��E�ֱ�ΪH��O��Cl����A��B��E�γɵĻ������ж��֣������й��ۼ����磺HClOΪ���ᣬ����D����
���Ա���𰸣�B��