��Ŀ����
����Ŀ��SCR��NSR��������Ч���Ͳ��ͷ������ڿ������������µ�NOx�ŷš�
��1��SCR��ѡ���Դ���ԭ������ԭ����
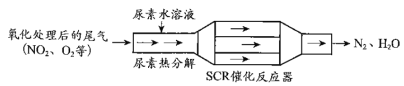
������[CO(NH2)2]ˮ��Һ�ȷֽ�ΪNH3��CO2����д��CO2�ĵ���ʽ___________________��
�ڷ�Ӧ����NH3��ԭNO2�����У�����ԭ���������������1mol��ת�Ƶ�������__________������NA��ʾ����
�۵�ȼ���к������ϸ�ʱ��β����SO2��O2�����»��γ�(NH4)2SO4��ʹ�����ж����û�ѧ����ʽ��ʾ(NH4)2SO4���γ�_________��
��������ҺŨ��Ӱ��NO2��ת�����ⶨ��Һ�����أ�M=60 g��mol1�������ķ������£�ȡa g������Һ������������ȫת��ΪNH3������NH3�ù�����v1 mL c1 mol��L1 H2SO4��Һ������ȫ��ʣ��H2SO4��v2 mL c2 mol��L1 NaOH��Һǡ���кͣ���������Һ�����ʵ�����������________
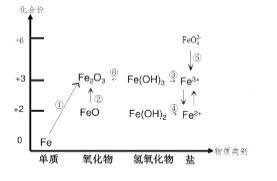
��2��NSR��NOx���滹ԭ������ԭ����NOx�Ĵ���ͻ�ԭ�ڲ�ͬʱ�ν�����У���ͼa��ʾ��
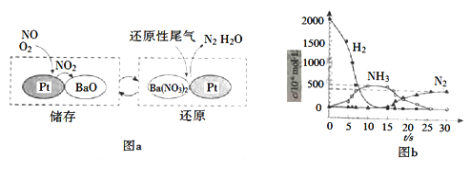
��ͨ��BaO��Ba(NO3)2���ת��ʵ��NOx�Ĵ���ͻ�ԭ������NOx��������_________��
����H2ģ��β���л�ԭ�������о���Ba(NO3)2�Ĵ���ԭ���̣��ù��̷��������У�ͼb��ʾ�ù����������Ũ����ʱ��ı仯��ϵ����һ����Ӧ���ĵ�H2��Ba(NO3)2�����ʵ���֮����___��
�ۻ�ԭ�����У���ʱ�����Ц����N2O������ͬλ��ʾ�ٷ��о�����Ц���IJ�����NO�йء�������������15NO��NH3��һ��������Ӧʱ���õ���Ц����������15NNO�����÷�Ӧ�Ļ�ѧ����ʽ����������_____________![]() 15NNO+H2O
15NNO+H2O
���𰸡�![]() 24NA 2SO2��O2��4NH3��2H2O===2(NH4)2SO4
24NA 2SO2��O2��4NH3��2H2O===2(NH4)2SO4 ![]() �� BaO 8��1 415NO��4NH3��3O2
�� BaO 8��1 415NO��4NH3��3O2
��������
(1)�ٶ�����̼Ϊ���ۻ��������C=O�������漰��ӦΪΪ8NH3+6NO2 ![]() 7N2+12H2O����Ϸ���ʽ�жϣ���SO2��O2��������NH3��H2O��Ӧ�γ�(NH4)2SO4���˷�Ӧ��SO2�ǻ�ԭ��������������������ϵ����غ��ԭ���غ�ɵô˷�Ӧ�Ļ�ѧ����ʽ�����漰��ӦΪ2NH3+H2SO4=(NH4)2SO4��2NaOH+H2SO4=Na2SO4+H2O���ɷ���ʽ��֪n(NaOH)+n(NH3)=2n(H2SO4)���Դ˼��㣻
7N2+12H2O����Ϸ���ʽ�жϣ���SO2��O2��������NH3��H2O��Ӧ�γ�(NH4)2SO4���˷�Ӧ��SO2�ǻ�ԭ��������������������ϵ����غ��ԭ���غ�ɵô˷�Ӧ�Ļ�ѧ����ʽ�����漰��ӦΪ2NH3+H2SO4=(NH4)2SO4��2NaOH+H2SO4=Na2SO4+H2O���ɷ���ʽ��֪n(NaOH)+n(NH3)=2n(H2SO4)���Դ˼��㣻
(2)����ͼa��֪����NOx��������BaO���ڵ�һ����Ӧ��H2����������ˮ�����ϼ���0�����ߵ�+1�ۣ�Ba(NO3)2��NԪ�ػ��ϼ���+5�۽��͵�-3�ۣ����ɰ�������ϵ�ʧ������Ŀ��ȼ��㣻��������������15NO��NH3��һ��������Ӧʱ���õ���Ц����������15NNO����NԪ���غ��֪15NO��NH3ӦΪ1��1����ϵ��ӵ�ʧ�����ƽ��
(1)�ٶ�����̼Ϊ���ۻ��������C=O��������ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��NH3��ԭNO2�������漰�ķ�ӦΪ8NH3+6NO2 ![]() 7N2+12H2O���ɷ���ʽ��֪����ԭ���������������1mol�������ɻ�ԭ����(N2)4mol����������(N2)3mol������8molNH3�μӷ�Ӧ��ת�Ƶ���24mol������Ϊ24NA���ʴ�Ϊ��24NA��
7N2+12H2O���ɷ���ʽ��֪����ԭ���������������1mol�������ɻ�ԭ����(N2)4mol����������(N2)3mol������8molNH3�μӷ�Ӧ��ת�Ƶ���24mol������Ϊ24NA���ʴ�Ϊ��24NA��
��SO2��O2��������NH3��H2O��Ӧ�γ�(NH4)2SO4���˷�Ӧ��SO2�ǻ�ԭ��������������������Ӧ�Ļ�ѧ����ʽΪ2SO2+O2+4NH3+2H2O�T2(NH4)2SO4���ʴ�Ϊ��2SO2+O2+4NH3+2H2O�T2(NH4)2SO4��
���漰��ӦΪ2NH3+H2SO4=(NH4)2SO4��2NaOH+H2SO4=Na2SO4+H2O����Ӧ��n(H2SO4)=v1��c1��10-3mol��n(NaOH)=v2��c2��10-3mol���ɷ���ʽ��֪n(NaOH)+n(NH3)=2n(H2SO4)����n(NH3)=(2v1��c1��10-3-v2��c2��10-3)mol����m(CO(NH2)2)=![]() ��(2v1��c1��10-3-v2��c2��10-3)mol��60g/mol=(0.06v1c1-0.03v2c2 )g��������Һ�����ʵ�����������
��(2v1��c1��10-3-v2��c2��10-3)mol��60g/mol=(0.06v1c1-0.03v2c2 )g��������Һ�����ʵ�����������![]() ��100%=
��100%=![]() %���ʴ�Ϊ��
%���ʴ�Ϊ��![]() %��
%��
(2)����ͼʾ��֪BaO��NOx��Ӧ����Ba(NO3)2��Ba(NO3)2�ٻ�ԭΪN2����NOx������ΪBaO���ʴ�Ϊ��BaO��
�ڵ�һ����Ӧ��H2����������ˮ�����ϼ���0�����ߵ�+1�ۣ�Ba(NO3)2��NԪ�ػ��ϼ���+5�۽��͵�-3�ۣ����ɰ�������1molBa(NO3)2���ɰ���ת��16mol���ӣ��μӷ�Ӧ�����������ʵ���Ϊ![]() =8mol�������ĵ�H2��Ba(NO3)2�����ʵ���֮����8��1���ʴ�Ϊ��8��1��
=8mol�������ĵ�H2��Ba(NO3)2�����ʵ���֮����8��1���ʴ�Ϊ��8��1��
��������������15NO��NH3��һ��������Ӧʱ���õ���Ц����������15NNO����NԪ���غ��֪15NO��NH3ӦΪ1��1����֪��Ӧ�Ļ�ѧ����ʽΪ415NO+4NH3+3O2 ![]() 415NNO+6H2O���ʴ�Ϊ��415NO+4NH3+3O2��
415NNO+6H2O���ʴ�Ϊ��415NO+4NH3+3O2��

����Ŀ�����в���Ԫ�ص�������ԭ�ӣ�����ӣ��ṹ���±���
Ԫ�ر�� | Ԫ��������ԭ�ӣ�����ӣ��ṹ |
T | �����������Ǵ�����������3�� |
X | �����µ���Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷ� |
Y | M���K����1������ |
Z | ��������Ԫ�صļ������а뾶��С |
��1��д��������Ԫ�ص�Ԫ�ط��ţ�T_______��X_______��Y_______��Z_______��
��2��д��Ԫ��T��ԭ�ӽṹʾ��ͼ_____________________________
��3��Ԫ��Y��Ԫ��Z��ȣ������Խ�ǿ����________����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ����__________������ţ���
��Y���ʵ��۵��Z���ʵ� ��Y�Ļ��ϼ۱�Z��
��Y������ˮ��Ӧ��Z���ʾ��� ��Y����������ˮ����ļ��Ա�Zǿ
��4��XԪ�ص���̬�⻯��������ۺ����ᷴӦ�ķ���ʽΪ______________________
��5��Ԫ��T����Ԫ����ԭ�Ӹ�����1��1�����γɻ�����Q��Ԫ��X����Ԫ����ԭ�Ӹ�����1��2�����γɳ��������ȼ�ϵĻ�����W��Q��W����������ԭ��Ӧ������X���ʺ�T����һ���⻯�д���÷�Ӧ�Ļ�ѧ����ʽ________________________________________