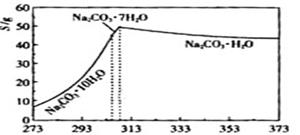
��Ŀ����
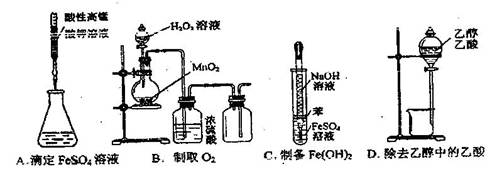
��������2008�������ѧ���в��ԣ���ѧ��5�����л�ѧʵ�������������ȷ����
| A����Һʱ��������Ȼ�̼��Һ�ӷ�Һ©���¿ڷų���ˮ����Ͽڵ��� |
| B������ʱ�����¶ȼ�ˮ�������ڱ������ʯ��Һ���£��Ҳ�����������ƿ�ĵײ� |
| C���ζ�ʱ�����ֿ��Ƶζ��ܻ����������ճ���ƿ���ߵα���ƽ�ӵζ�����Һ�� |
| D������ʱ����������ڳ���ֽ������������ƽ�����̣����������������ƽ������ |
A
���ڵ�����Ȼ�̼��Һ���ܶȱ�ˮ���ܶȴʷ�Һʱ���ܶȴ�ĵ�����Ȼ�̼��Һ�ӷ�Һ©���¿ڷų���ˮ����Ͽڵ���������A����ȷ������ʱ���¶ȼƲ�����ʱ�������¶ȣ��ʽ��¶ȼ�ˮ��������������ƿ��֧�ܴ�������B����ζ�ʱ�����ֿ��Ƶζ��ܻ����������ճ���ƿ���۾�ע������ƿ����Һ����ɫ�仯����C�������ڴ���ˮ��ʼ��ԣ����и�ʴ�ԣ�����ʱӦ���ڲ��������У���D�����

��ϰ��ϵ�д�
�����Ŀ

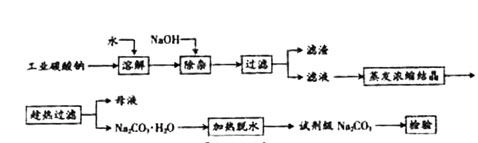
 �����ʣ��ᴿ����·�����£�
�����ʣ��ᴿ����·�����£�