��Ŀ����
����Ŀ��������(��HR ��ʾ)��һ�ָ�Ч������������һ��Ũ�ȵ���������Һȥ��ˮ������Һ��H3R��H2R-��HR2-��R3-�ĺ�����pH�ı仯��ͼ��ʾ������˵����ȷ����
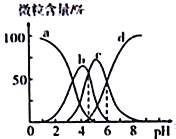
A. H3R�ĵڶ������볣��Ka2(H3R)��������Ϊ10-4
B. pH=6ʱ��c(R3-)=c(HR2-)>c(H+)>c(OH-)
C. Na2HR��Һ��HR2-��ˮ��̶ȴ��ڵ���̶�
D. pH=4ʱ��c(H+)=c(H2R-)+2c(HR2-)+c(OH-)
���𰸡�B
��������A. ��ͼ��֪����pH��������H3R��H2R-��HR2-��R3-��ͼ���ж�Ӧ�����߷ֱ�Ϊa��b��c��d��H3R�ĵڶ������볣��Ka2(H3R)=![]() ����ͼ��֪����c(HR2��)= c(H2R��)ʱ��pHԼΪ4.8��c(H+)=10��4.8mol/L��������ӦΪ10��5����A����B. ��ͼ��֪��pH=6ʱ��c(R3-)=c(HR2-)>c(H+)>c(OH-)����B��ȷ��C. HR2-�������ڵ���ҺpHԼΪ5����Һ�����ԣ�����Na2HR��Һ��HR2-��ˮ��̶�С�ڵ���̶�����C����D. pH=4ʱ����Һ�е������ӳ�H+���Ca2����Mg2���ȣ����Բ����ϵ���غ㣬��D����ѡB��
����ͼ��֪����c(HR2��)= c(H2R��)ʱ��pHԼΪ4.8��c(H+)=10��4.8mol/L��������ӦΪ10��5����A����B. ��ͼ��֪��pH=6ʱ��c(R3-)=c(HR2-)>c(H+)>c(OH-)����B��ȷ��C. HR2-�������ڵ���ҺpHԼΪ5����Һ�����ԣ�����Na2HR��Һ��HR2-��ˮ��̶�С�ڵ���̶�����C����D. pH=4ʱ����Һ�е������ӳ�H+���Ca2����Mg2���ȣ����Բ����ϵ���غ㣬��D����ѡB��

��ϰ��ϵ�д�
�����Ŀ


