��Ŀ����
A��B��C��X����ѧ��ѧ�������ʣ�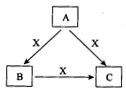 ���ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�
���ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�
��1����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���A��C��ˮ
��Һ��Ͽɵ�B�ij���
��A��B��C�к��е�ͬһ�ֳ�������Ԫ��Ϊ___________��
��д��A��C��ˮ��Һ������ɳ���B�����ӷ�Ӧ����ʽ
Ϊ ��
��2����AΪ��̬�ǽ������ʣ�A��XͬΪ��������Ԫ�أ����³�ѹ��CΪ��ɫ���壬B�����и�ԭ��������Ϊ8e![]() �ṹ��
�ṹ��
��B�ĵ���ʽΪ_____________��
��C����ˮ���ҷ�Ӧ��������������ᣬ��Ӧ�Ļ�ѧ����ʽΪ_____________![]() ��
��
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��ˮ��Һ��Ϊ���ԣ���C�ӵ������У�����ɫ��ζ������X������
��A�������еĻ�ѧ����_____________��
�ڽ�����Xͨ��ˮ������Һ�У�д����Ӧ�����ӷ���ʽ___________��
����Ȼ���д���B��C��H2O��һ�������ᾧ���ɵĹ��塣ȡһ�����ù�������ˮ���100mL��Һ����������н��������ӵ�Ũ��Ϊ0��5 mol��L����ȡ��ͬ�����Ĺ�����������أ�ʣ����������Ϊ__________ g��
��1����Al ��Al3++3AlO2-+6H2O=4Al(OH)3
��2����![]() ��PCl5+4H2O=H3PO4+5HC1��
��PCl5+4H2O=H3PO4+5HC1��
��3�������Ӽ� ���ۼ�
�� 2H2O+2CO2+SiO32��=H2SiO3��+2HCO3����
�� 2.65 g

 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�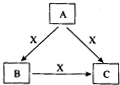 A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺
A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺ A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�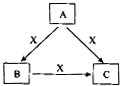 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�
 A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺ A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺