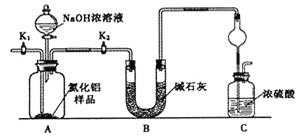
��Ŀ����
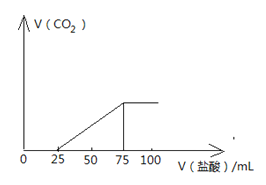
����Ŀ���о����֣�����Խϡ����ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡ�����������Ͻ���������ϡ�������ַ�Ӧ��û������ų����ڷ�Ӧ���������Һ�У���μ���4 mol/LNaOH ��Һ������NaOH��Һ�������mL��������ij��������ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ������˵������ȷ����

A. O����Һ�е�������Ϊ��H+��Fe2+��Fe3+��Al3+
B. DE�η�Ӧ�����ӷ���ʽΪ��NH4++OH-=NH3��H2O
C. �Ͻ��У�n(Al)=0.008mol
D. ���ⶨF�������������ʵ�鲽���ǣ����ˡ�ϴ�ӡ��������
���𰸡�A
��������A����ͼ��֪��OC�η�����������кͣ�˵�������������Һ�в�������Fe2+����A����B��DE�εμӵ�NaOH��Һ����Һ�е�NH4+��Ӧ����Ӧ�����ӷ���ʽΪNH4++OH-=NH3��H2O����B��ȷ��C����ͼ��֪����EF�η���Al��OH��3+OH-=AlO2-+2H2O����DE�����ĵ�NaOH��Һ���Ϊ2mL����֪n[Al��OH��3]=4.0mol/L��0.002L=0.008mol��������Ԫ���غ㣬�ʻ�Ͻ�����n��Al��=0.008mol����C��ȷ��D�����ⶨF�������������Ӧ���������ܵĹ�����Һ����룬��ϴ�ӳ������ŵ��ӻ����ٸ����������ʵ�鲽���ǣ����ˡ�ϴ�ӡ������������D��ȷ����ΪA��

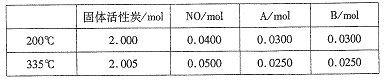
����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬���б���ʵ������������ȷ����
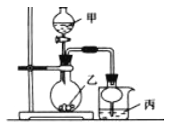
ʵ�� | �Լ��� | �Լ��� | �Լ��� | ���е����� |
A | 70%���� | Na2SO3 | ��ɫʯ����Һ | ��Һ�ȱ�����ɫ |
B | Ũ���� | KMnO4 | ����̪��NaOH��Һ | ��Һ��ɫ |
C | ϡ���� | CaCO3 | BaCI2��Һ | �а�ɫ�������� |
D | Ũ��ˮ | CaO | AlCl3 | �����ɰ�ɫ����Ȼ������ܽ� |
A. A B. B C. C D. D
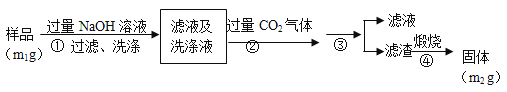
����Ŀ��ˮ�ϼ�ʽ̼��þ[4MgCO3��Mg(OH)2��4H2O],�ֳ�����̼��þ����������þ������������Ϊ̼��þ90%,̼���10%��̼������ȡ��
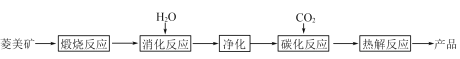
��1���������б�������,ѡ��������Ӧ������¶�__________;������___________________________��
�����¶�/�� | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
ת����/% | 50.3 | 58.2 | 85.1 | 85.2 | 90.7 | 91.2 | 91.5 |
��2��̼����Ӧ������Mg(HCO3)2,����Mg(HCO3)2�Ļ�ѧ����ʽΪ____________��
��3������ͼ��__________��__________����Ϊ̼����Ӧ�ṩ������̼Դ��
��4���й����������±ˮ̼������ȡ����̼��þ��
��±ˮ�к���Fe2+��Mn2+,����Ũ��С��1��10-5mol��L-1ʱ,������Ϊ��ȫ��ȥ������ʱ��������ҺPHΪ9.5ʱ,��ʱMn2+С��__________mol��L-1,�������������
���� | Mg(OH)2 | Fe(OH)3 | Fe(OH)2 | Mg(OH)2 |
Ksp | 10-11 | 10-39 | 10-17 | 10-14 |
�������Fe2+ת��ΪFe3+,�ӻ����Ƕ�ѡ������ʵ�������Ϊ__________��
A��Ca(ClO)2 B��Cl2 C��H2O2 D��HNO3
�����з�����,�Ϻõ�Ϊ__________��������______________________��
![]()