��Ŀ����
����ʯ��Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ���H2SO4��Ӧ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
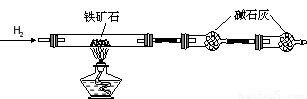
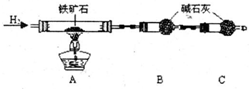
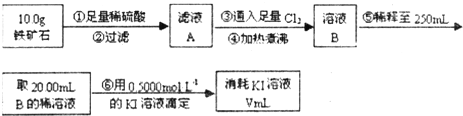
???????????? A????????????? B???????? C
��������ʯ�к������IJⶨ
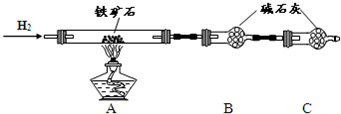
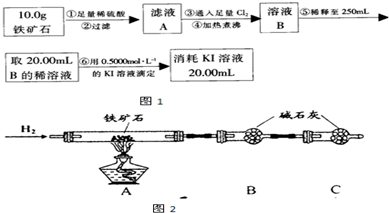
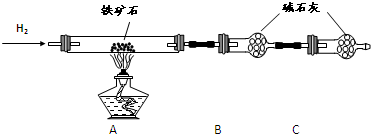
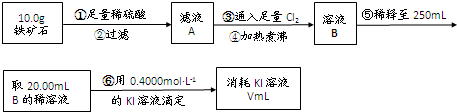
�� ����ͼ��װ���������װ�õ������ԣ�?
�� ��5.0g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
�� ����˵����ܿڴ�����ͨ��H2��____________����ȼA���ƾ���
�� ��ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ����������ȫ��ȴ��
��1��װ��C������Ϊ________________________________________________��
��2�����е�ȼA���ƾ���ǰ�������Ϊ��______________________________ ��
��3����÷�Ӧ��װ��B����1.35g��������ʯ�����İٷֺ���Ϊ____________��
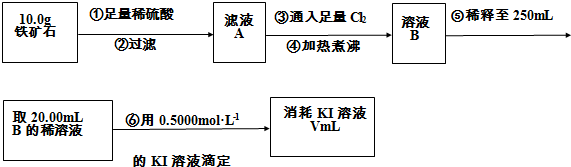
��������ʯ�к������IJⶨ
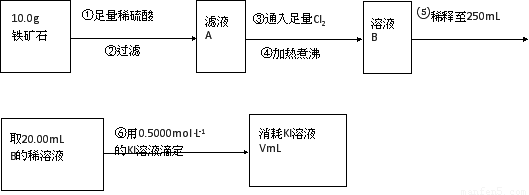
��1������������������__________________________________________��
��2�����������õ��IJ����������ձ�������������ͷ�ιܡ�____________��
��3�������йز������IJ�����˵����ȷ����__________________��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b���ζ������п����õ�����Һ��Ϊָʾ��
c���ζ���������ˮϴ�Ӻ����ֱ��װҺ
d����ƿ����Ҫ�ô���Һ��ϴ
e���ζ������У��۾�ע�ӵζ�����Һ��仯
f���ζ���ɫ�仯��30s����Һ���ָ�ԭ������ɫ�ٶ���
��4�����ζ�����������0.5000mol��L?1KI��Һ20.00mL��������ʯ�����İٷֺ���Ϊ____________��
���������������������������ʯ������������Ļ�ѧʽΪ???????????????????????????????? ��
��μ�����ҺA���Ƿ���Fe2+__________����ѡ����ĸ����
A.�ȼ�KSCN��Һ���ټ���ˮ??? B.��NaOH��Һ??? C.��K3[Fe(CN)6]
������1����ֹ�����е�ˮ������CO2����B�У�Ӱ��ⶨ�����
��2�����������Ĵ���??? ��3��24%?
������1��������Һ���ܽ�Ĺ�����Cl2?
��2��250mL����ƿ?? ��δ����250mL�������֣���3��df??? ��4��70%??
��. Fe5O6? C
��������
���������
������1��B�еļ�ʯ���������û���Ӧ���ɵ�ˮ�ģ�Ϊ�˷�ֹ�����ɷֶ�ʵ���Ӱ�죬Ҫ��һ��װ�����տ����е�ˮ�ֺͶ�����̼
��2����ȼ����֮ǰҪ�ȼ��������Ĵ��ȣ��Է���ը
��3����Ӧ��װ��B����1.35g������������������Ӧ���������������ֵ�����Ը��ݲ����������㣻��Ӧʵ�ʣ����ӵ�����Ԫ�ص�������������Ԫ�ص����������ǣ�1.35/18)��16/5.0��100%=24%
����
��1����п��Խ�ˮ�е�������ߣ�
��2������ϡ��Һ�������һ���������Һ��ѡ����������ش�����ƿ��һ�ֶ���������
��3�����ݵζ������Լ��ζ������е�ʵ��������֪ʶ���ش�
��ˮΪ��ɫ������������Ҳ�ǻ�ɫ��Һ���ζ���������Ҫ��ָʾ����a����
�ζ������У����������Ժ͵����ӷ�����Ӧ�����������Ӻ͵ⵥ�ʣ��ⵥ������������Һ��ʾ��ɫ�������������ӵ���ɫ������������ã�b����
�ζ���������ˮϴ�Ӻ�������ñ�Һϴ�ӣ�c����
�ζ������У��۾�Ӧע������ƿҺ���з����ı仯
��4������Ԫ���غ�ͻ�ѧ��Ӧ����ʽ���м���
������ȡ��Һ����Fe3+�������������KI��Һ�������ȣ���Ϸ���ʽ��֪��c(Fe3+��=c(KI)=0.5mol/L,������Ԫ�صİٷֱȺ���Ϊ70%
��.������Ԫ��������������Ԫ�������������������������Ļ�ѧʽ����������������70%����Ԫ�ص�����������24%������100g����ʯ�У���Ԫ�ص�������70g����Ԫ�ص�������24g����Ԫ�غ���Ԫ�ص����ʵ�����Ϊ��70/50������24/16��=5��6������������Ļ�ѧʽΪFe5O6
���㣺����Ի�������������ռ�ʵ�����