��Ŀ����
��2013?����һģ������ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ���H2SO4��Ӧ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
������ʯ�к������IJⶨ
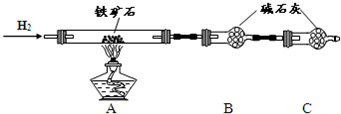
�ٰ���ͼ��װ���������װ�õ������ԣ�
�ڽ�5.0g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
�۴���˵����ܴ����ϵػ���ͨ��H2����Cװ�ó��ڴ�H2�鴿��ȼA���ƾ���
�ܳ�ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ����������ȫ��ȴ��
��1��װ��C������Ϊ
��2����ķ�Ӧ��װ��B����1.35g��������ʯ�����İٷֺ���Ϊ
������ʯ�к������IJⶨ
��1�����������������
��2����������õ��IJ����������ձ�������������ͷ�ιܡ�
��3�������йز���IJ�����˵����ȷ����
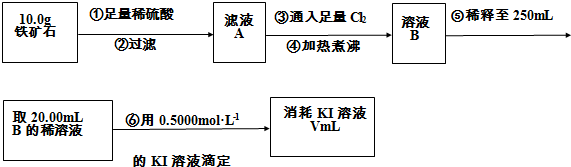
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b���ζ������п����õ�����Һ��Ϊָʾ��
c���ζ���������ˮϴ�Ӻ����ֱ��װҺ
d����ƿ����Ҫ�ô���Һ��ϴ
e���ζ������У��۾�ע�ӵζ�����Һ��仯
f���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ���
��4�����ζ�����������0.5000mol?L-1 KI��Һ20.00mL��������ʯ�����İٷֺ���Ϊ

������ʯ�к������IJⶨ
�ٰ���ͼ��װ���������װ�õ������ԣ�
�ڽ�5.0g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
�۴���˵����ܴ����ϵػ���ͨ��H2����Cװ�ó��ڴ�H2�鴿��ȼA���ƾ���
�ܳ�ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ����������ȫ��ȴ��
��1��װ��C������Ϊ
��ֹ�����е�ˮ������CO2����B�У�Ӱ��ⶨ���
��ֹ�����е�ˮ������CO2����B�У�Ӱ��ⶨ���
����2����ķ�Ӧ��װ��B����1.35g��������ʯ�����İٷֺ���Ϊ
24%
24%
��������ʯ�к������IJⶨ
��1�����������������
������Һ���ܽ�Ĺ�����Cl2
������Һ���ܽ�Ĺ�����Cl2
����2����������õ��IJ����������ձ�������������ͷ�ιܡ�
250mL����ƿ
250mL����ƿ
����3�������йز���IJ�����˵����ȷ����
df
df
��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b���ζ������п����õ�����Һ��Ϊָʾ��
c���ζ���������ˮϴ�Ӻ����ֱ��װҺ
d����ƿ����Ҫ�ô���Һ��ϴ
e���ζ������У��۾�ע�ӵζ�����Һ��仯
f���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ���
��4�����ζ�����������0.5000mol?L-1 KI��Һ20.00mL��������ʯ�����İٷֺ���Ϊ
70%
70%
����������1��B�еļ�ʯ���������û���Ӧ���ɵ�ˮ�ģ�Ϊ�˷�ֹ�����ɷֶ�ʵ���Ӱ�죬Ҫ��һ��װ�����տ����е�ˮ�Լ�������̼����2����Ӧ��װ��B����1.35g������������������Ӧ���������������ֵ�����Ը��ݲ����������㣻
��1����п��Խ�ˮ�е�������ܣ���2������ϡ��Һ�������һ���������Һ��ѡ����������ش𣻣�3�����ݵζ������Լ��ζ������е�ʵ��������֪ʶ���ش��жϣ���4������Ԫ���غ�ͻ�ѧ��Ӧ����ʽ���м��㣮
��1����п��Խ�ˮ�е�������ܣ���2������ϡ��Һ�������һ���������Һ��ѡ����������ش𣻣�3�����ݵζ������Լ��ζ������е�ʵ��������֪ʶ���ش��жϣ���4������Ԫ���غ�ͻ�ѧ��Ӧ����ʽ���м��㣮
����⣺��1����ʵ���У���������������Ӧ���ɽ�������ˮ�����ݹ��������ı仯���������ĺ�����B���ĸ�������������ղ�����ˮ����������Cװ��Ҫ��ֹ��ֹ�����е�ˮ������CO2����B�У�Ӱ��ⶨ������ʴ�Ϊ����ֹ�����е�ˮ������CO2����B�У�Ӱ��ⶨ�������2����ķ�Ӧ��װ��B����1.35g�����ݷ�Ӧ��ʵ�ʣ����ӵ�����Ԫ�ص�������������Ԫ�ص����������ǣ�
��100%=24%���ʴ�Ϊ��24%��
��1��������ʯ�м������ᣬ����Ӧ���������������Һ�������ڹ�����������Һ�������������������е������Ǹ�����Һ���ܽ�Ĺ�����Cl2���ʴ�Ϊ��������Һ���ܽ�Ĺ�����Cl2��
��2������ƿ��һ�ֶ�������������ϡ�͵�250mL������õ������У��ձ�������������ͷ�ιܡ�250mL����ƿ���ʴ�Ϊ��250mL����ƿ��
��3��a����ˮΪ��ɫ������������Ҳ�ǻ�ɫ��Һ���ζ����������ָʾ������a����
b���ζ������У����������Ժ͵����ӷ�����Ӧ�����������Ӻ͵ⵥ�ʣ��ⵥ������������Һ��ʾ��ɫ�������������ӵ���ɫ������������ã���b����
c���ζ���������ˮϴ�Ӻ�����ñ�Һ��ϴ����c����
d����ƿ����Ҫ�ô���Һ��ϴ����d��ȷ��
e���ζ������У��۾�ע����ƿ����ɫ�ı仯����e����
f���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ�������f��ȷ��
��ѡdf��
��4��������ȡ��Һ����Fe3+�������������KI��Һ�������ȣ���Ϸ���ʽ��֪��c��Fe3+��=c��KI��=0.5mol?L-1��������Ԫ�صİٷֺ���Ϊ��
��100%=70%���ʴ�Ϊ��70%��
| ||
| 5.0 |
��1��������ʯ�м������ᣬ����Ӧ���������������Һ�������ڹ�����������Һ�������������������е������Ǹ�����Һ���ܽ�Ĺ�����Cl2���ʴ�Ϊ��������Һ���ܽ�Ĺ�����Cl2��
��2������ƿ��һ�ֶ�������������ϡ�͵�250mL������õ������У��ձ�������������ͷ�ιܡ�250mL����ƿ���ʴ�Ϊ��250mL����ƿ��
��3��a����ˮΪ��ɫ������������Ҳ�ǻ�ɫ��Һ���ζ����������ָʾ������a����
b���ζ������У����������Ժ͵����ӷ�����Ӧ�����������Ӻ͵ⵥ�ʣ��ⵥ������������Һ��ʾ��ɫ�������������ӵ���ɫ������������ã���b����
c���ζ���������ˮϴ�Ӻ�����ñ�Һ��ϴ����c����
d����ƿ����Ҫ�ô���Һ��ϴ����d��ȷ��
e���ζ������У��۾�ע����ƿ����ɫ�ı仯����e����
f���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ�������f��ȷ��
��ѡdf��
��4��������ȡ��Һ����Fe3+�������������KI��Һ�������ȣ���Ϸ���ʽ��֪��c��Fe3+��=c��KI��=0.5mol?L-1��������Ԫ�صİٷֺ���Ϊ��
| 0.5mol/L��0.25L��56g/mol |
| 10g |
��100%=70%���ʴ�Ϊ��70%��
������������һ��̽�����ʵ���ɡ��������ʵĺ���֪ʶ��һ���ۺϿ����⣬����ѧ�������ͽ��������������ۺ���ǿ���Ѷȴ�

��ϰ��ϵ�д�
�����Ŀ
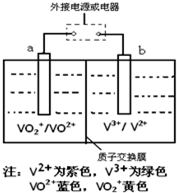 ��2013?����һģ��ij����ص�ԭ����ͼ��ʾ����Һ��c��H+��=2.0mol?L-1��������ΪSO42-��a��b��Ϊ���Ե缫�����ʱ�Ҳ���Һ��ɫ����ɫ��Ϊ��ɫ�����жԴ˵��������ȷ���ǣ�������
��2013?����һģ��ij����ص�ԭ����ͼ��ʾ����Һ��c��H+��=2.0mol?L-1��������ΪSO42-��a��b��Ϊ���Ե缫�����ʱ�Ҳ���Һ��ɫ����ɫ��Ϊ��ɫ�����жԴ˵��������ȷ���ǣ�������