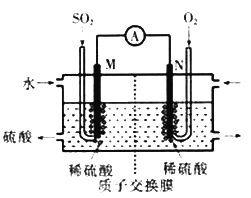
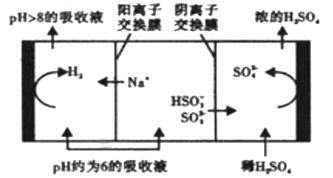
��Ŀ����
����Ŀ������ʵ�ˮ��Һ�д��ڵ���ƽ�⣮
��1�������dz��������ᣮ
�� ������ˮ��Һ�еĵ��뷽��ʽΪ________________________________________________��
�����з����У�����ʹ����ϡ��Һ��CH3COOH����̶��������_________������ĸ��ţ� ��
A���μ�����Ũ���� B������Һ C����ˮϡ�� D���������������ƾ���
��2����0.1 molL-1 NaOH��Һ�ֱ�ζ������Ϊ20.00 mL��Ũ�Ⱦ�Ϊ0.1 molL-1������ʹ�����Һ���õ��ζ���������ҺpH�����NaOH��Һ������仯�������ζ����ߡ�

�� ������������______________������I����������
�� �ζ���ʼǰ��������Һ����ˮ�������c(H+)������_____________________��
�� V1��V2�Ĺ�ϵ��V1 _____ V2��������������=��������������
���𰸡� CH3COOH![]() CH3COO��+H+ B��C I 0.1 mol��L-1������Һ <
CH3COO��+H+ B��C I 0.1 mol��L-1������Һ <
�����������⿼��������ʵĵ��룬�Լ��ζ�ʵ�飬��1���ٴ��������ᣬӦ�Dz��ֵ��룬CH3COOH![]() CH3COO����H������A���������ᣬ���ƴ���ĵ��룬����̶Ƚ��ͣ���A����B��������ʵĵ��������ȹ��̣������¶ȣ�ƽ��������Ӧ������У�����ĵ���̶�����B��ȷ��C����ˮϡ�ͣ��ٽ����룬�������̶�����C��ȷ��D����������ƣ�CH3COO��Ũ���������ƴ���ĵ��룬����̶ȼ�С����D����2���ٴ��������ᣬ������ǿ�ᣬ��ʼʱ�����pHС�ڴ����pH����I�ǵζ����ᣬII�ǵζ�����������������������ǿ����ʣ���ˮ�ĵ�������ǿ�ڴ��ᣬ��˵ζ�ǰ��0.1mol��L��1CH3COOH��Һ��ˮ�������c(H��)���CH3COOH�����ᣬ�ζ���pH=7����Һ�е�����ΪCH3COOH��CH3COONa��������ǿ�ᣬ�ζ���pH=7ʱ������ΪNaCl������������NaOH�����С����������NaOH�������V1<V2��
CH3COO����H������A���������ᣬ���ƴ���ĵ��룬����̶Ƚ��ͣ���A����B��������ʵĵ��������ȹ��̣������¶ȣ�ƽ��������Ӧ������У�����ĵ���̶�����B��ȷ��C����ˮϡ�ͣ��ٽ����룬�������̶�����C��ȷ��D����������ƣ�CH3COO��Ũ���������ƴ���ĵ��룬����̶ȼ�С����D����2���ٴ��������ᣬ������ǿ�ᣬ��ʼʱ�����pHС�ڴ����pH����I�ǵζ����ᣬII�ǵζ�����������������������ǿ����ʣ���ˮ�ĵ�������ǿ�ڴ��ᣬ��˵ζ�ǰ��0.1mol��L��1CH3COOH��Һ��ˮ�������c(H��)���CH3COOH�����ᣬ�ζ���pH=7����Һ�е�����ΪCH3COOH��CH3COONa��������ǿ�ᣬ�ζ���pH=7ʱ������ΪNaCl������������NaOH�����С����������NaOH�������V1<V2��

����Ŀ���±���ѡ���У����������û���Ӧͨ��Y�õ�W��һ�黯�������� ��
ѡ�� ������ | A | B | C | D |
Y | H2O | FeCl3 | H2O | C |
W | HF | CuCl2 | Fe3O4 | Si |
A. A B. B C. C D. D