��Ŀ����
����Ŀ��ʵ���ҴӺ����Һ����H2O�⣬����CCl4��I2��![]() �ȣ��л��յ⣬ʵ��������£�
�ȣ��л��յ⣬ʵ��������£�

��1�����Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ_______���ò�����I2��ԭΪI����Ŀ����___________________��
��2������![]() ������Ϊ__________��
������Ϊ__________��
��3������ʱ��������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����40�����ҷ�Ӧ��ʵ��װ����ͼ��ʾ����ʵ������ڽϵ��¶��½��е�ԭ����__________________������a������Ϊ________������b��ʢ�ŵ���ҺΪ__________��

��4����֪��![]() ��ij�����ˮ��pHԼΪ8����һ������I2�����ܴ���I����IO3���е�һ�ֻ����֡��������������麬���ˮ���Ƿ���I����IO3����ʵ�鷽����ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ����
��ij�����ˮ��pHԼΪ8����һ������I2�����ܴ���I����IO3���е�һ�ֻ����֡��������������麬���ˮ���Ƿ���I����IO3����ʵ�鷽����ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ����
��ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�
��_______________________________________________________________________________��
������ˮ����ȡ������Һ������1-2mL������Һ���������ữ�μ�Na2SO3��Һ������Һ����˵����ˮ�к���IO3��������˵����ˮ�в�����IO3����
��5���������ȣ�ClO2������ɫ������ˮ�����壩�Ǹ�Ч���Ͷ�����������ˮ������������ClO2�������Ժ���Һ���յ⡣���ClO2����I�������ӷ���ʽ��________________________________��
��6��������������һ�ֲⶨS2����������Ч���������·�ZnS��BaSO4��һ�ֳ��õİ�ɫ���ϣ��Ʊ������л��������Ե�BaS�������������������ⶨ���·���Ʒ��S2���ĺ�������ȡm g��Ʒ�����ڵ���ƿ�У���ȡ25.00 mL 0.1000 mol/L ��I2-KI��Һ�����У�������������Һ���ܱգ��ð�����Ӧ5min���е������������Ե���Ϊָʾ����������I2��0.1000 mol/L Na2S2O3 �ζ�����ӦʽΪI2 + 2S2O32��=2I��+ S4O62�����ⶨ����Na2S2O3��Һ���V mL�����·���ƷS2������Ϊ__________��д������ʽ��
���𰸡�SO32-+I2+H2O=2I-+2H++SO42- ʹCCl4�еĵ����ˮ�� ��Һ ʹ��������Һ���нϴ���ܽ�� ���������� NaOH��Һ ��ˮ����ȡ������Һ������1~2mL������Һ�����������ữ���μ��Ȼ�����Һ������Һ����ɫ��˵����ˮ�к�I-������I- 2ClO2+10I-+8H+=5I2+2Cl-+4H2O 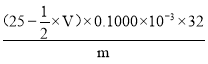 ��100%
��100%
��������
�������Һ�м����������ƣ�����Һ�е�I2��ԭΪI-�����Ȼ�̼���ܶȱ�ˮ�����²㣬�÷�Һ�ķ������룬Ȼ�����ϲ���Һ�м�ǿ�������������������ӣ����ͨ���������������ռ��ⵥ�ʣ��ﵽ�����ᴿ��Ŀ�ġ�
(1)����������ԣ������������������������ƣ���������ԭ����I-��������ˮ����������������ˮ��
(2)���Ȼ�̼������ˮ��
(3)�������������������ܽ�������¶ȵ����߶���С������������״�����÷����ش��������ƣ����������������ܺ�����������Һ��Ӧ��
(4)�����Ӿ��л�ԭ�ԣ��ܱ��������������ɵ⣬�ⵥ���������۱���ɫ��
(5)��ClO2�������Ժ�I-��Һ���յ⣬�Ƕ�������������Һ���������������ɵⵥ�ʣ��������ȱ���ԭΪ�����ӣ�
(6)����ת�Ƶ����غ�֪��ϵʽS2-��I2�����I2 +2S2O32��=2I��+ S4O62����������Na2S2O3��Ӧ���ĵ�n(I2)��������������ӷ�Ӧ�ĵ⣬�Ӷ����S2�������ʵ�������������Ʒ��S2��������
(1)����������ԣ������������������������ƣ���������ԭ���ɵ����ӣ����ӷ�Ӧ����ʽΪSO32-+I2+H2O=2I-+2H++SO42-��������ˮ����������������ˮ��Ϊ��ʹ�����IԪ�ؽ���ˮ��ҺӦ���ԭΪ�����ӣ��ʴ�Ϊ��SO32-+I2+H2O=2I-+2H++SO42-��ʹ���Ȼ�̼�еĵ����ˮ�㣻
(2)���Ȼ�̼�����л��ˮ����������߲����ܣ����뻥�����ܵ�Һ��������÷�Һ�ķ��������Է�������Ȼ�̼���÷�Һ�ķ������ʴ�Ϊ����Һ��
(3)�������������������ܽ�������¶ȵ����߶���С���¶�Խ�ߣ��������ܽ��ԽС����ӦԽ����֣�����Ӧ���ڵ��������½��з�Ӧ������ͼʾ������aΪ���������ܣ����������������ж�������ֱ���ſգ����߶��ܺ�����������Һ��Ӧ�������գ�������NaOH��Һ����β�����ʴ�Ϊ��ʹ��������Һ���нϴ���ܽ�ȣ����������ܣ�NaOH��Һ��
(4)�����Ӿ��л�ԭ�ԣ��ܱ��������������ɵ⣬��������Ӿ��������ԣ��ܱ���ԭ����ԭ���ɵ⣬����������Һ����ɫ����������鷽��Ϊ����ˮ��ȡ������Һ������1-2mL������Һ�����������ữ���μ�FeCl3��Һ��2I-+2Fe3+=2Fe2++I2������Һ����ɫ��˵����ˮ�к���I-������I-���ʴ�Ϊ����ˮ��ȡ������Һ������1-2mL������Һ�����������ữ���μ�FeCl3��Һ������Һ����ɫ��˵����ˮ�к���I-������I-��
(5)��ClO2�������Ժ�I-��Һ���յ⣬��������������Һ���������������ɵⵥ�ʣ��������ȱ���ԭΪ�����ӣ���Ӧ�����ӷ���ʽΪ��2ClO2+10I-+8H+=5I2+2Cl-+4H2O���ʴ�Ϊ�� 2ClO2+10I-+8H+=5I2+2Cl-+4H2O��
(6)��Na2S2O3��Ӧ���ĵ�n(I2)=![]() n(Na2S2O3)=
n(Na2S2O3)=![]() ��0.1000molL-1��V��10-3 L=
��0.1000molL-1��V��10-3 L=![]() ��0.1000��V��10-3 mol�����������ӷ�Ӧ��n(I2)=25��10-3 L��0.1000molL-1-
��0.1000��V��10-3 mol�����������ӷ�Ӧ��n(I2)=25��10-3 L��0.1000molL-1-![]() ��0.1000��V��10-3 mol=(25��10-3 ��0.1000-
��0.1000��V��10-3 mol=(25��10-3 ��0.1000-![]() ��0.1000��V��10-3)mol������ת�Ƶ����غ�֪��ϵʽS2-��I2������n(S2-)=(25��10-3 ��0.1000-
��0.1000��V��10-3)mol������ת�Ƶ����غ�֪��ϵʽS2-��I2������n(S2-)=(25��10-3 ��0.1000-![]() ��0.1000��V��10-3)mol��m(S2-)=(25��10-3 ��0.1000-
��0.1000��V��10-3)mol��m(S2-)=(25��10-3 ��0.1000-![]() ��0.1000��V��10-3)mol��32g/mol������������=
��0.1000��V��10-3)mol��32g/mol������������= ��100%=
��100%=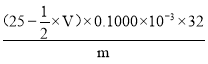 ��100%���ʴ�Ϊ��
��100%���ʴ�Ϊ��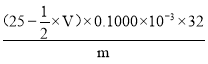 ��100%��
��100%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij�������Ʒ�л���������李���ȡ��ͬ��������Ʒ�ֱ���100mL 2.300mol/L������������Һ��ַ�Ӧ���õ���ʵ���������±���
ʵ����� | �� | �� |
��Ʒ������g�� | 14.35 | 28.70 |
����������g�� | 3.570 | 3.570 |
����˵���������
A. ��Ʒ���������������淋����ʵ���֮��Ϊ9��1
B. ʵ�������������һ������
C. Ҫʹʵ������Ʒ��ȫ��Ӧ����Ҫ�ټ�����������0.2100mol
D. ��Ʒ�е�Ԫ�ص�����������20.49%
����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵��ǣ� ��

ѡ�� | �� | �� | �� | ʵ����� |
A | Ũ���� | KMnO4 | ��ɫʯ����Һ | �����������ԡ�Ư���� |
B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� |
C | ϡ���� | Na2SO3 | Ʒ����Һ | ʵ������ȡ������SO2 |
D | Ũ���� | Na2CO3 | Na2SiO3��Һ | �ǽ����ԣ�N��C��Si |
A.AB.BC.CD.D









