��Ŀ����
ijУ�о���ѧϰС���ͬѧ�Խ���þ�ڿ�����ȼ�յIJ������̽����
��1����ͬѧ��Ϊ�������д��ڴ����ĵ�����þ���ܺ͵�����Ӧ�������������װ�ý���ʵ�飺

þ��ȼ�����ȡ��������۲죬�����������г��а�ɫ������������ĵ���ɫ���塣��֧�ּ�ͬѧ�۵��ʵ����������ɵ���ɫ�����⣬���е�������__________________��������_________________________________________��
��2����ͬѧ�Լ�ͬѧʵ�������ɵĵ���ɫ�����������Ȥ�������������еĹ���μ�����ˮ�������д̼�����ζ���������������ɫ����ת��ɰ�ɫ������д�����������з����Ļ�ѧ��Ӧ����ʽ��_______________________________________��
��1�������е�ˮ��������������ݻ���1��5 ��
��Ϊ������O2���������ԼΪ1��5��CO2�����������С��ֻ�к�N2��Ӧ�������е�ˮ���������ܴ�������ݻ���1��5
��2��Mg3N2��6H2O��3Mg(OH)2��2NH3��

��ϰ��ϵ�д�
�����Ŀ

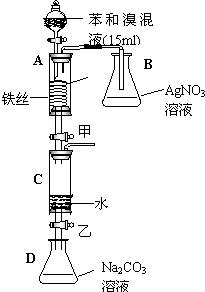 ��ͼ��ijУ�о���ѧϰС���ͬѧ��Ƶ��йر���������������ʱ��Ӧ��ʵ��װ��ͼ����ش��������⣺
��ͼ��ijУ�о���ѧϰС���ͬѧ��Ƶ��йر���������������ʱ��Ӧ��ʵ��װ��ͼ����ش��������⣺