��Ŀ����
����Ŀ����1����������4�����ʵ������գ���O2 ��NH4NO3 ��K2O2 ��NH3���������Ӽ����м��Լ�����___________��������ţ�
��2���Ȼ������������ʷdz����⣬�磺�Ȼ������۵�Ϊ190 ��(2.02��105 Pa)������180 ���Ϳ�ʼ�������ݴ��жϣ��Ȼ�����___________(�������ۻ��������������ӻ�������)�����ʵ��֤������ж���ȷ��ʵ��������________________��
��3������a��g7�ֶ�����Ԫ�أ����������ڱ��е�λ�����£���ݴ˻ش��������⣺

��Ԫ�ص�ԭ�Ӽ䷴Ӧ�������γ����Ӽ�����___________��
A��c��f B��b��g C��d��g D��b��e
��d��gԪ���γɵķ���������ԭ��___________(������������������)�����������Ϊ8���ӽṹ��
��4��A��B��C��D���ֶ�����Ԫ�أ����ǵ�ԭ������������������A��C��B��D�ֱ���ͬ����Ԫ�أ���֪B��D��Ԫ�ؼ���γ�DB2��DB3���ֻ����������Է����������16������A��C��Ԫ��ԭ������֮����B��D��Ԫ��ԭ������֮�͵�1/2����ش�����������
��д����B��C����Ԫ���γɵ�ԭ�Ӹ�����Ϊ1:1�Ļ�����ĵ���ʽ��________���侧����������ѧ����������________��
��A2B��A2D�ķе㣺A2B________A2D(��������������������)����ԭ����____________��
����A��B��C��D����Ԫ���γɵ�����X�������ᷴӦ�ܹ����ɾ��д̼�����ζ�����壬д��X�����ᷴӦ�����ӷ���ʽ��____________________________��
��̼Ԫ�ص�һ��ͬλ�ؿɲⶨ�������������ͬλ�صķ�����________��
���𰸡� �� ���ۻ����� �Ȼ���������״̬�²��ܵ��� B �� ![]() ���Ӽ������ۼ����Ǽ��Թ��ۼ��� ���� ˮ���Ӽ���������������Ӽ�û����� HSO3-+H+��SO2��+H2O
���Ӽ������ۼ����Ǽ��Թ��ۼ��� ���� ˮ���Ӽ���������������Ӽ�û����� HSO3-+H+��SO2��+H2O ![]()
����������1��һ����õĽ����ͻ��õķǽ��������γ����Ӽ����ǽ���Ԫ�ص�ԭ�Ӽ������γɹ��ۼ���ͬ�ַǽ����γɷǼ��Լ�����ͬ�ǽ����γɼ��Լ����ݴ��жϣ�
��2���Ȼ������۵�Ϊ190�棨2.02��105 Pa��������180��Ϳ�ʼ��������֪�۷е�ͣ�����Ӿ�����������ƣ�
��3����Ԫ�ص�λ�ÿ�֪��aΪH��bΪNa��cΪMg��dΪC��eΪN��fΪP��gΪCl��
��4����B��D��Ԫ�ؼ���γ�DB2��DB3���ֻ����������Է����������16����֪BΪOԪ�أ�B��D�ֱ���ͬ����Ԫ�أ�����DΪSԪ�أ�B��D�γɵĻ�����ΪSO2��SO3��S��OԪ��ԭ������֮��Ϊ24����A��CԪ��ԭ������֮��Ϊ12����A��Cͬ���壬����AΪHԪ�أ�CΪNaԪ�أ�����Ԫ�ض�Ӧ�ĵ��ʡ�����������ʽ��Ԫ�������ɽ��
��1����O2�����к��зǼ��Լ�����NH4NO3�к������Ӽ��ͼ��Լ�����K2O2�к������Ӽ�����ԭ�Ӻ���ԭ��֮�仹�зǼ��Լ�����NH3������ֻ�м��Լ�����������Ӽ����м��Լ����ǹ������أ���ѡ����
��2���Ȼ������۵�Ϊ190�棨2.02��105 Pa������180��Ϳ�ʼ��������֪�۷е�ͣ�����Ӿ�����������ƣ���֪�ɹ��ۼ��γɵĹ��ۻ����ʵ���������Ȼ���������״̬�²��ܵ��硣
��3����Ԫ�ص�λ�ÿ�֪��aΪH��bΪNa��cΪMg��dΪC��eΪN��fΪP��gΪCl����
�ٻ��õĽ�������õķǽ��������γ����Ӽ�����Ԫ�ص�ԭ�Ӽ䷴Ӧ�������γ����Ӽ�����b��g��NaCl������ΪB��
��d��gԪ���γɵ�CCl4����������ԭ�Ӷ����������Ϊ8���ӽṹ��
��4���������Ϸ�����֪A��B��C��D�ֱ���H��O��Na��S����
����B��C����Ԫ���γɵ�ԭ�Ӹ�����Ϊ1:1�Ļ������ǹ������ƣ�����ʽΪ![]() ���侧����������ѧ�������������Ӽ����ۼ���
���侧����������ѧ�������������Ӽ����ۼ���
��H2O��H2S�����⻯�����嶼���ڷ��Ӿ��壬���Ӿ��������ʵķе�������Է������������ȣ���ˮ�к�������������в�����������Զ��߷е�ϸߵ���H2O��
��A��B��C��D����Ԫ���γɵ�����XΪNaHSO3��NaHSO3�����ᷴӦ���ɵĶ���������д̼�����ζ����Ӧ�����ӷ���ʽΪHSO3-+H+��SO2��+H2O��
��̼Ԫ�ص�һ��ͬλ�ؿɲⶨ�������������ͬλ�صķ�����![]() ��
��

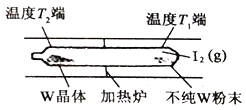
����Ŀ����һ�����¶ȡ�ѹǿ�ͷ��������ڵ������£�SO2�������е�O2����ΪSO3��V2O5�Ƿ������Ļ��Գɷ֣������͵������V2O5�ڶԷ�ӦI�Ĵ�ѭ�������У������ˢ�������Ӧ�Σ�ͼʾ��ͼ1��
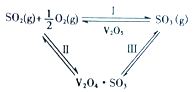
ͼ1
��1������֪�й����������1mol��ѧ������ʱ��Ҫ���յ������������£�
��ѧ�� | S=O(SO2) | O=O(O2) | S=O(SO3) |
����/kJ | 535 | 496 | 472 |
�ɴ˼��㷴Ӧ��ġ�H=_________kJ��mol-1��
��д����Ӧ��Ļ�ѧ����ʽ_________��
��2������˵����Ӧ��ﵽƽ��״̬����_________��
A�������ܱ������л�������ѹǿ���ٱ仯
B���������������л��������ܶȲ��ٱ仯
C���������������ʵ������ٱ仯
D����������ƽ����Է����������ٱ仯
E��n(SO2)��n(O2)��n(SO3)=2��1��2
F��SO2����İٷֺ������ٱ仯
��3���ڱ�����ϵ��ѹΪ105Pa�������½��з�ӦSO2+1/2O2![]() SO3��ԭ������SO2��O2�����ʵ���֮��m(m=
SO3��ԭ������SO2��O2�����ʵ���֮��m(m=![]() )��ͬʱ��SO2��ƽ��ת�������¶�(t)�Ĺ�ϵ����ͼ��ʾ��
)��ͬʱ��SO2��ƽ��ת�������¶�(t)�Ĺ�ϵ����ͼ��ʾ��
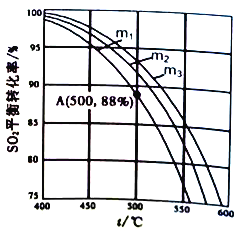
��ͼ��m1��m2��m3�Ĵ�С˳��Ϊ_________��������_________��
�ڷ�ӦI�Ļ�ѧƽ�ⳣ��Kp����ʽΪ_________(��ƽ���ѹ����ƽ��Ũ�ȱ�ʾ)��ͼ��A��ԭ�����ijɷ��ǣ�n(SO2)=10mol,n(O2)=24.4mol,n(N2)=70mol,��ƽ��ʱSO2�ķ�ѹp(SO2)Ϊ_________Pa��(��ѹ=��ѹ�����ʵ�������)��
�۽��꣬�����з�������������������¹��գ�ʹSO2����ȫ��ת�����˹��յ��ŵ�����ܳ�����ú����ԭ���⣬��Ҫ����_________��