��Ŀ����
����Ŀ��������������һ�ֹ���������Ƽ����ܱ�ҶƬ�Ͽ�����գ�����ֲ�����ڴ����ٶȽ���������ѿǰ���ݼ�����Ҫ���ڴ��ݵȡ���ҵ��ͨ������A���кϳɣ���ϳ�·�����£�

��֪��![]()
![]() RCH=O+H2O(R��������)
RCH=O+H2O(R��������)
(1)�Լ���Ϊ��_____________���Լ���Ϊ___________��
(2)д��A�Ľṹ��ʽ_____________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ����Ӧ��____________����Ӧ��____________��
(4)���ڱ����Ͳ������ŵ��Ӱ�죬�����������Ļ����ڱ�����ȡ����λ����ԭ�л��ž������磺���ӷ�������![]() ��ʹ������_________
��ʹ������_________![]() ѡ�����գ���ͬ
ѡ�����գ���ͬ![]() ��Hԭ�����ױ�ȡ����������Ŀ��Ϣ��֪��
��Hԭ�����ױ�ȡ����������Ŀ��Ϣ��֪��![]() ��ʹ������________��Hԭ�����ױ�ȡ����
��ʹ������________��Hԭ�����ױ�ȡ����
![]() ��λ
��λ ![]() ��λ
��λ ![]() ��λ
��λ
(5)��Ӧ�����ڱ���![]() ��Һ�н��У�����NaOH��Һ�н��У�����һ��ˮ�⣬��д��
��Һ�н��У�����NaOH��Һ�н��У�����һ��ˮ�⣬��д��![]() ������NaOH��Һ����ȫˮ��Ļ�ѧ��Ӧ����ʽ_________________
������NaOH��Һ����ȫˮ��Ļ�ѧ��Ӧ����ʽ_________________
���𰸡����� �״� ![]()
![]()
![]()

![]()
![]() +HNO3
+HNO3![]()
![]() +H2O
+H2O ![]() b
b ![]()
![]()

![]()
��������
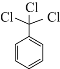
����A�ڹ�������������![]() ����֪AΪ
����֪AΪ![]() ���ٽ��D��
���ٽ��D��![]() ����
����![]() ��֪DΪ
��֪DΪ![]() ��C�����ᣬ���������µĵ�D����֪CΪ��
��C�����ᣬ���������µĵ�D����֪CΪ��![]() ������C����BΪ
������C����BΪ ���Լ�
���Լ�![]() Ϊ�״���
Ϊ�״���
![]() �ɷ�����֪�Լ�
�ɷ�����֪�Լ�![]() Ϊ�������Լ�
Ϊ�������Լ�![]() Ϊ�״���
Ϊ�״���
�ʴ�Ϊ��Ϊ������Ϊ�״���
![]() �ɷ�����֪A�Ľṹ��ʽ
�ɷ�����֪A�Ľṹ��ʽ![]() ��
��
�ʴ�Ϊ��![]() ��
��
![]() �ɷ�����֪����ʽΪ��
�ɷ�����֪����ʽΪ��![]()
![]()
![]()
![]()
![]() ��
��![]() +HNO3
+HNO3![]()
![]() +H2O��
+H2O��
�ʴ�Ϊ��![]()
![]()
![]()

![]() ��
��![]() +HNO3
+HNO3![]()
![]() +H2O��
+H2O��
![]() ���ڱ����Ͳ������ŵ��Ӱ�죬�����������Ļ����ڱ�����ȡ����λ����ԭ�л��ž������磺���ӷ�������
���ڱ����Ͳ������ŵ��Ӱ�죬�����������Ļ����ڱ�����ȡ����λ����ԭ�л��ž������磺���ӷ�������![]() ��ʹ�����ϵ��ڶ�λ��û��ã��ɷ�Ӧ
��ʹ�����ϵ��ڶ�λ��û��ã��ɷ�Ӧ![]() ��֪��
��֪��![]() ��ʹ�����ϼ�λ��Hԭ�����ױ�ȡ����
��ʹ�����ϼ�λ��Hԭ�����ױ�ȡ����
�ʴ�Ϊ��ac��b��
![]() ��Ӧ
��Ӧ![]() ����NaOH��Һ�н��л�һ�����ɱ������ƣ�����ʽΪ��
����NaOH��Һ�н��л�һ�����ɱ������ƣ�����ʽΪ��![]() +4NaOH��
+4NaOH��![]()
![]() ��
��
�ʴ�Ϊ��![]() +4NaOH��
+4NaOH��![]()
![]() ��
��

 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д�����Ŀ����������Ԫ�أ�����A��B��C��D��EΪ��������Ҫ��Ԫ�أ�FΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
AԪ���γɵ���������࣬���γɵ�һ�ֹ��嵥�ʹ�ҵ�ϳ������и�� |
BԪ��ԭ�ӵĺ���p��������s��������1 |
CԪ�ػ�̬ԭ��p���������δ�ɶԵ��� |
Dԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� ��1=738kJ��mol-1����2=1451kJ��mol-1����3=7733kJ��mol-1����4=10540kJ��mol-1�� |
Eԭ�Ӻ�������p���ȫ������� |
F�����ڱ��ĵ�8���� |
��1��ijͬѧ����������Ϣ���ƶ�A��̬ԭ�ӵĺ��������Ų�Ϊ��![]() ����ͬѧ�����ĵ����Ų�ͼΥ����___________��
����ͬѧ�����ĵ����Ų�ͼΥ����___________��
��2��BԪ�صĵ縺��_____������ڡ�����С�ڡ����ڡ���CԪ�صĵ縺�ԡ�
��3��C��D�γɵĻ����������еĻ�ѧ������Ϊ_____________��
��4��E��̬ԭ����������ߵĵ��ӣ���������ڿռ���__________������
��5�����й���Fԭ�ӵļ۲�����Ų�ͼ��ȷ����___________��
a.![]() b.
b.![]()
c.![]() d.
d.![]()
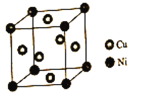
��6����̬F3+���Ӻ�������Ų�ʽΪ_____________����������F��B������������ˮ�����ϡ��Һ��ȫ��Ӧ������BC���壬�÷�Ӧ�����ӷ���ʽΪ____________��
��7��Ԫ��ͭ�����ĵڶ������ֱܷ�Ϊ��ICu=1959kJ��mol-1,INi=1753kJ��mol-1,ICu��INiԭ����__________________��
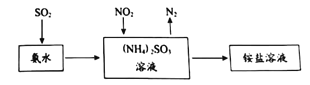
����Ŀ��ij��ѧ��ѧ��ȤС��Ϊ�˵��鵱��ijһ������ˮ����Ⱦ�������ע�������3����ҪˮԴ����ڴ��ɼ�ˮ�����������˷���������������ʵ����Ϣ������һ��ˮԴ����A��B�������ʣ�һ������C��D�������ʣ�һ������E���ʣ�A��B��C��D��EΪ���ֳ�������������±��е������γɣ�
������ | K+ Na+ Cu2+ Al3+ |
������ | SO42�� HCO3- NO3- OH�� |
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ��ֻ��B��CΪ��ɫ������ɫ�ܲ�������
���ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬA�зų���ɫ���壬C��D�ж��ܲ�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��������ʵ����գ�
��1��д��C��D���Ļ�ѧʽ��C_______��D______��
��2������1 mol A����Һ�뺬l mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ������д��A��E��Ӧ�����ӷ���ʽ��_______________��
��3����A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ_____________________��
��4��C��������ˮ���������ӷ���ʽ���ʵ�����˵���侻ˮԭ��______________________��
��5����������0.5 mol��C��Һ����μ���Ba(OH)2��Һ�����ɳ����������Ϊ__________g��