��Ŀ����
| |||||||||||||||
������
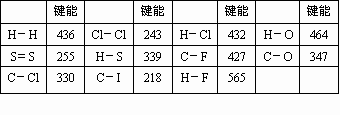
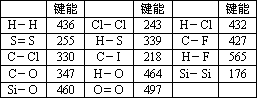
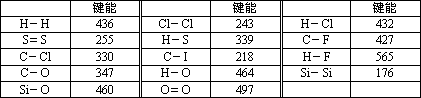
(1) |
����,����,218~330 KJ��mol��1 |
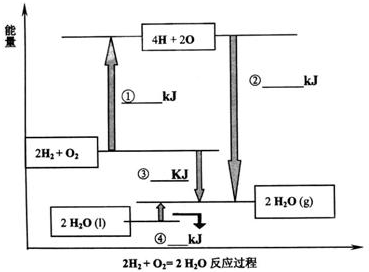
(2) |
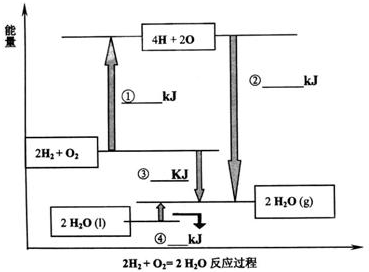
������ѧ��Ӧ�ķ�Ӧ�ȵ���������ļ���֮���뷴Ӧ��ļ���֮�͵IJ�; ��������,4.5 |
(3) |
������FeO(s)��CO(g)��Fe(s)��CO2(g)����H����11 kJ��mol��1; �����ڶ���һЩ������ʵ�鷽��ֱ�Ӳⶨ�����Ļ�ѧ��Ӧ���á�Hess���ɡ���Ӳⶨ�� |

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�