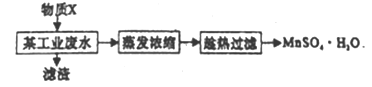
��Ŀ����
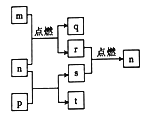
����Ŀ����ҵ�ϳ������̿���Ҫ�ɷ�ΪMnO2���Ʊ�KMnO4,��������������ʾ��

(1)��ԭ�Ϸ����ͨ�˿�������������ʱ��������Ӧ�Ļ�ѧ����ʽΪ_______________��
(2)���̿��г��˺���MnO2��������Fe2O3��MgO��Al2O3��SiO2������,��Щ��־�ᵼ��KOH������______
���ƫ�ߡ���ƫ�͡�)��
(3)���������п���ѭ��ʹ�õ�������MnO2��_____________��д��ѧʽ����
(4)��Ӧa�Ļ�ѧ����ʽΪ________________��
(5)����I������Ϊ_________������II����KMnO4��K2CO3�ܽ��ԵIJ��죬��ȡ________(������������ƣ������ȹ��˵õ�KMnO4���־��塣�־���ϴ�Ӻ�õ������ľ��塣֤�������Ѿ�ϴ�Ӹɾ��IJ�����______��
(6)ij�����ŷŵķ�ˮ�к���Mn2+��Ca2+��Mg2+��������ΪSO42-)�����뽫Mn2+�������ã��������������̣�
��֪��

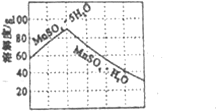
����XӦѡ��__________(��һ�������Ӻ�һ����������ɣ��ѧʽ���������ܽ�����߿�֪����������Ũ�������ȹ��˲���ʱ����Ƶ��¶�������Ϊ___________������ţ���
��20�� ��40�� ��80�� ��120��
���𰸡� 4KOH+2MnO2+O2![]() 2K2MnO4+2H2O ƫ�� KOH 3 K2MnO4+2CO2�T2 KMnO4+ MnO2��+2K2CO3 ���� Ũ���ᾧ ȡ���һ��ϴ��Һ���Թ��У��μ�CaCl2��Һ����û�г������ɣ�˵����ϴ�� MnF2 ��
2K2MnO4+2H2O ƫ�� KOH 3 K2MnO4+2CO2�T2 KMnO4+ MnO2��+2K2CO3 ���� Ũ���ᾧ ȡ���һ��ϴ��Һ���Թ��У��μ�CaCl2��Һ����û�г������ɣ�˵����ϴ�� MnF2 ��
����������1����������ͼ�м���������Լ����������ʣ��õ���Ӧ�ķ���ʽ��KOH+MnO2+O2��K2MnO4������������ԭ��Ӧ��ȱ����ƽ��OH-ת���ˮ��KOH+MnO2+O2��K2MnO4+H2O��Mn��+4������Ϊ+6����2������������0�۽�����-2�۽�4����С��������4��MnO2��K2MnO4ϵ����2��O2ϵ����1������ԭ���غ���ƽ��������4KOH+2MnO2+O2![]() 2K2MnO4+2H2O����2�����̿��л���Al2O3��SiO2��Al2O3�������Ժͷ�Ӧ��2KOH+ Al2O3=2KAlO2+H2O ��SiO2 �����������������кͷ�Ӧ��SiO2+2KOH��K2SiO3+H2O���������ĵ�KOHƫ�ߣ���3�������������֪�����������п���ѭ��ʹ�õ�������MnO2��KOH����4�����ݷ�Ӧa.3K2MnO4+2CO2=2KMnO4+MnO2+2K2CO3��3molMn���2mol KMnO4��������
2K2MnO4+2H2O����2�����̿��л���Al2O3��SiO2��Al2O3�������Ժͷ�Ӧ��2KOH+ Al2O3=2KAlO2+H2O ��SiO2 �����������������кͷ�Ӧ��SiO2+2KOH��K2SiO3+H2O���������ĵ�KOHƫ�ߣ���3�������������֪�����������п���ѭ��ʹ�õ�������MnO2��KOH����4�����ݷ�Ӧa.3K2MnO4+2CO2=2KMnO4+MnO2+2K2CO3��3molMn���2mol KMnO4��������![]() ��100%��66.7%����5���������ǽ����ܵ�MnO2���룬�ù��˵ķ���������KMnO4��K2CO3�������ýᾧ�ķ������������������ܽ���ϵIJ��죬����Ũ���ᾧ�����ȹ��˵õ�KMnO4�־��壻�־�����溬������̼��أ�֤�������Ѿ�ϴ�Ӹɾ��IJ�����ȡ���һ��ϴ��Һ���Թ��У��μ�CaCl2��Һ����û�г������ɣ�˵����ϴ����(6) ���ڲ����������ʣ���������ѡ�������ӣ�����ΪҪ������Һ�е�þ���Ӻ����ӣ���X���ܶȻ���������̫С�����Ը��ݱ������ݿ�֪���ӵ�����ΪMnF2��Ϊ��֤�����ľ���ΪMnSO4��H2O���ұ�������Խϵ��¶���MnSO4��H2O�ܽ⣬������¶���40�����ϣ���Խ��Խ�����ھ������������ֲ��ܸ���100��(ˮ��Һ����¶�) ����ѡ�ۡ�
��100%��66.7%����5���������ǽ����ܵ�MnO2���룬�ù��˵ķ���������KMnO4��K2CO3�������ýᾧ�ķ������������������ܽ���ϵIJ��죬����Ũ���ᾧ�����ȹ��˵õ�KMnO4�־��壻�־�����溬������̼��أ�֤�������Ѿ�ϴ�Ӹɾ��IJ�����ȡ���һ��ϴ��Һ���Թ��У��μ�CaCl2��Һ����û�г������ɣ�˵����ϴ����(6) ���ڲ����������ʣ���������ѡ�������ӣ�����ΪҪ������Һ�е�þ���Ӻ����ӣ���X���ܶȻ���������̫С�����Ը��ݱ������ݿ�֪���ӵ�����ΪMnF2��Ϊ��֤�����ľ���ΪMnSO4��H2O���ұ�������Խϵ��¶���MnSO4��H2O�ܽ⣬������¶���40�����ϣ���Խ��Խ�����ھ������������ֲ��ܸ���100��(ˮ��Һ����¶�) ����ѡ�ۡ�

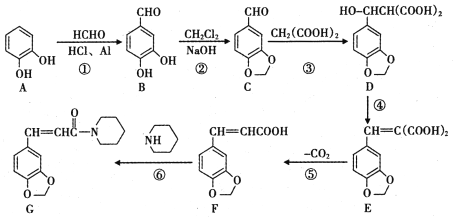
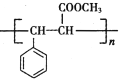
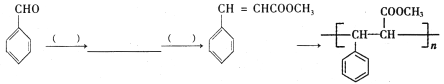
����Ŀ�� ���ǵ�ѭ�������е���Ҫ���ʣ����ĺϳ���Ŀǰ�ձ�ʹ�õ��˹��̵�������
��1��������ͼ�ṩ����Ϣ��д���÷�Ӧ���Ȼ�ѧ����ʽ
_____________________________________________��
��ͼ��������____________���a�� ��b������ʾ ��������ý���������������仯���ߡ�
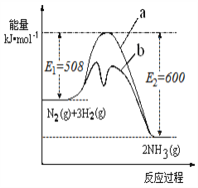
��2���ں��������У�������������˵��������Ӧ�Ѵ�ƽ�����_________________��
A��3��(H2)����2��(NH3)��
B����λʱ��������n mol N2��ͬʱ����2n molNH3
C��������������ܶȲ���ʱ��ı仯���仯
D��������ѹǿ����ʱ��ı仯���仯
��3��Ϊ��Ѱ�Һϳ�NH3������������ijͬѧ���������ʵ�飨���±����������±��ո�������Ӧ��ʵ�����������ݡ�
ʵ���� | T(��) | n (N2)/n(H2) | P(MPa) |
�� | 450 | 1/3 | 1 |
�� | ______ | 1/3 | 10 |
�� | 480 | ______ | 10 |
��4���ϳɰ���ԭ���������Ʊ�ʱ����һ����Ӧ�� H2O(g)��CO(g)![]() CO2(g)��H2(g)����850 ��ʱ����ƽ�ⳣ��K��1����850 ��ʱ�����Ϊ1 L�ĺ����ܱ������У�ͬʱ����1.0 mol CO,3.0 mol H2O,1.0 mol CO2,5.0 mol H2����ʱ��Ӧ��____________(�����Ӧ�����淴Ӧ��)������У�ƽ��ʱCO2�����ʵ���Ϊ______________��
CO2(g)��H2(g)����850 ��ʱ����ƽ�ⳣ��K��1����850 ��ʱ�����Ϊ1 L�ĺ����ܱ������У�ͬʱ����1.0 mol CO,3.0 mol H2O,1.0 mol CO2,5.0 mol H2����ʱ��Ӧ��____________(�����Ӧ�����淴Ӧ��)������У�ƽ��ʱCO2�����ʵ���Ϊ______________��