��Ŀ����
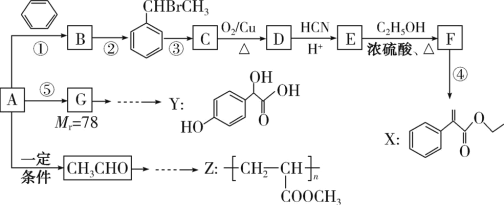
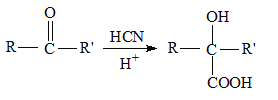
����Ŀ������������ֲ�����ڵ����ȩ��һ�־���ɱ�������������õ��л�������������ϳ����Ϲ�ҵ�����E����ˮ�Ը߷��Ӿ���N��·��ʾ��ͼ��
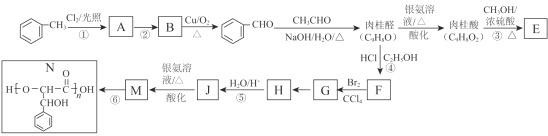
��֪����R-CHO+CH3CHO![]() R-CH=CH=CHO(RΪ����)
R-CH=CH=CHO(R����)
��RCHO![]() RCH(OC2H5)2
RCH(OC2H5)2![]() RCHO
RCHO
(1)�ٵķ�Ӧ������__________����������Լ���������__________��
(2)��Ȼ������Ȼ���ڵ����ȩ��Ϊ��ʽ�ṹ����ṹ��__________��
(3)�۵Ļ�ѧ����ʽ��__________��
(4)M��������������ͬ�����ŵ�������__________��
(5)�ϳ�·���Тܡ������������Ŀ����__________��
(6)P��E��ij��ͬ���칹�壬д����������������P�Ľṹ��ʽ_____________________��
a.��������ͬ�Ĺ����ţ�������������Һ����������
b.�����ں��������˴Ź�������������壬�����֮����2:2:1
���𰸡�ȡ����Ӧ NaOH��Һ������ ![]()
![]() �ǻ� ����ȩ�������뷴Ӧ
�ǻ� ����ȩ�������뷴Ӧ ![]()
��������
�ױ�����ȡ����Ӧ����A��A��Ӧ����B��B������������Ӧ���ɱ���ȩ����B�ṹ��ʽΪ![]() ��A�ṹ��ʽΪ
��A�ṹ��ʽΪ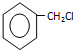 ������ȩ����ȩ���������Ϣ�ķ�Ӧ�������ȩ�����ȩ�ṹ��ʽΪ
������ȩ����ȩ���������Ϣ�ķ�Ӧ�������ȩ�����ȩ�ṹ��ʽΪ![]() �����ȩ����������ӦȻ���ữ�õ�����ᣬ�����ṹ��ʽΪ�����ȩ������Ϣ�ķ�Ӧ����F��F�ṹ��ʽΪ
�����ȩ����������ӦȻ���ữ�õ�����ᣬ�����ṹ��ʽΪ�����ȩ������Ϣ�ķ�Ӧ����F��F�ṹ��ʽΪ![]() �������ͼ״�����������Ӧ����E��E�ṹ��ʽΪ
�������ͼ״�����������Ӧ����E��E�ṹ��ʽΪ![]() �����ȩ������Ϣ�ķ�Ӧ֪��F�ṹ��ʽΪ
�����ȩ������Ϣ�ķ�Ӧ֪��F�ṹ��ʽΪ![]() ��F�����ӳɷ�Ӧ����G��G�ṹ��ʽΪ
��F�����ӳɷ�Ӧ����G��G�ṹ��ʽΪ![]() ��G����ˮ�ⷴӦ����H��H�ṹ��ʽΪ
��G����ˮ�ⷴӦ����H��H�ṹ��ʽΪ![]() ��H��������������ˮ������J��J�ṹ��ʽΪ
��H��������������ˮ������J��J�ṹ��ʽΪ ��J����������Ӧ����M��M�ṹ��ʽΪ
��J����������Ӧ����M��M�ṹ��ʽΪ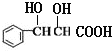 ���ݴ˷������
���ݴ˷������
1)ͨ�����Ϸ���֪���ٵķ�Ӧ������ȡ����Ӧ����������Լ���������NaOH��ˮ��Һ�����ȣ�
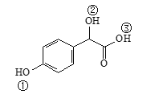
(2)��Ȼ������Ȼ���ڵ����ȩ��Ϊ��ʽ�ṹ����ṹ��![]() ��
��
(3)�۵Ļ�ѧ����ʽ��![]() ��
��
(4)M�ṹ��ʽΪ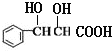 ��M��������������ͬ�����ŵ��������ǻ���
��M��������������ͬ�����ŵ��������ǻ���
(5)�ϳ�·���Тܡ������������Ŀ���DZ���ȩ�������뷴Ӧ��
(6)E�ṹ��ʽΪ![]() ��P��E��ij��ͬ���칹�壬P�Ľṹ��ʽ�������������� a����������ͬ�Ĺ����ţ�������������Һ������������˵������ȩ����b�������ں��������˴Ź�������������壬�����֮����2��2��1�����������Ľṹ��ʽΪ
��P��E��ij��ͬ���칹�壬P�Ľṹ��ʽ�������������� a����������ͬ�Ĺ����ţ�������������Һ������������˵������ȩ����b�������ں��������˴Ź�������������壬�����֮����2��2��1�����������Ľṹ��ʽΪ![]() ��
��

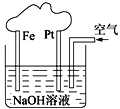
����Ŀ����þ������þ��������ɰ����(Na2B4O7��10H2O)ʱ�ķ���������Ҫ�ɷ���MgO��������Na2B4O7��CaO��Fe2O3��FeO��MnO��SiO2�����ʡ�����þ��Ϊԭ����ȡ��ˮ����þ�Ĺ����������£�

�ش��������⣺
(l)Na2B4O7��10H2O��B�Ļ��ϼ�Ϊ__________��
(2)Na2B4O7������ˮ��Ҳ����ˮ�⣺B4O72-+7H2O![]() 4H3BO3(����)+2OH-(�����ڳ������ܽ�Ƚ�С)��д����������ʱNa2B4O7������Ӧ�Ļ�ѧ����ʽ��______________��
4H3BO3(����)+2OH-(�����ڳ������ܽ�Ƚ�С)��д����������ʱNa2B4O7������Ӧ�Ļ�ѧ����ʽ��______________��
(3)����B�к��в�����ϡ���ᵫ��������Ũ����ĺ�ɫ���壬д�����ɺ�ɫ��������ӷ���ʽ____________��
(4)����MgO��Ŀ����___________________��
(5)��֪MgSO4��CaSO4���ܽ�����±���
�¶�(��) �ܽ��(g) | 40 | 50 | 60 | 70 |
MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
������A���ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ�������ϱ����ݣ���Ҫ˵��������A������Ϊ____________________��
(6)��ɰҲ���ڹ�ҵ����ȡNaBH4��NaBH4����Ϊ�л���ѧ�еġ����ܻ�ԭ������
��д��NaBH4�ĵ���ʽ��___________��
�ڡ���Ч�⺬�����������������ԭ���Ļ�ԭ�������䶨���ǣ�ÿ�˺��ԭ���Ļ�ԭ�����൱�ڶ��ٿ�H2�Ļ�ԭ������NaBH4����Ч�⺬��Ϊ_________��������λС������
���ڼ��������£��������ϵ��NaBO2Ҳ���Ƶ����⻯�ƣ�д�������ҵĵ缫��Ӧʽ��________��