��Ŀ����
��ˮ��������ʱ������˫����(H2Dz����Ԫ����)�ѽ���������ϳɵ����Ե����ʣ�����CCl4��ȡ�����Ӷ��ѽ������Ӵ�ˮ��Һ����ȫ�������������˫����(H2Dz)��CCl4������ˮ�е�Cu2��ʱ���ȷ�����Ϸ�Ӧ��Cu2��+2H2Dz(1)д��˫�����Fe3����ϵ����ӷ���ʽ��________________________����ȡ������Ҫ�������˵���ȡ������Һ��pH����������________________________��
��ͼ����˫����(H2Dz)��CCl4�����ȡijЩ�������ӵ�������ߡ�����ӳ����ȡijЩ��������ʱ���˵�pH��Χ��E%��ʾij�ֽ����������������ʽ����ȡ����İٷ��ʡ�

ij��ҵ��ˮ�к���Hg2����Bi3+��Zn2+����˫����(H2Dz)��CCl4�����ȡ��������ˮ��
�������ͼ�ش����⣺
(2)����ȫ����ˮ�е�Hg2������������������ҺpH=____________��
(3)������pH=2ʱ����(Bi)�Ĵ�����ʽ��____________�������ʵ���֮��Ϊ__________��
(4)��ȡ��CCl4�е�Zn(HDz)2��Һ����������NaOH��Һ�������п��ת��ˮ��Һ�С�д����Ӧ�����ӷ���ʽ��________________________��
(1)Fe3++3H2Dz![]() Fe(HDz)3+3H+ Fe3+���γ�Fe(OH)3����
Fe(HDz)3+3H+ Fe3+���γ�Fe(OH)3����
(2)1
(3)Bi3+��Bi(HDz)3 3��2
(4)Zn(HDz)2+6OH-![]()
![]() +2Dz2-+2H2O
+2Dz2-+2H2O
������(1)��˫����ѽ���������ϳɵ����Ե�����֪��Fe3++3H2Dz![]() Fe(HDz)3+3H+���������pH�����Fe3+��ת��ΪFe(OH)3������
Fe(HDz)3+3H+���������pH�����Fe3+��ת��ΪFe(OH)3������
(2)��ͼ�ɿ���pH=1ʱHg2+ȫ�����������ʽ����ȡ���������
(3)��ͼ֪pH=2ʱ��Bi3+���������ʽBi(HZ2)3������ȡ�İٷ�����40%������n(Bi3+)��n��Bi(HZ2)3��=60%��40%=3��2��
(4)Zn(HDz)2+6OH-![]()
![]() +2Dz2-+2H2O
+2Dz2-+2H2O



 Cu (HDZ)2+2H+���ټ���CCl4
��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
Cu (HDZ)2+2H+���ټ���CCl4
��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�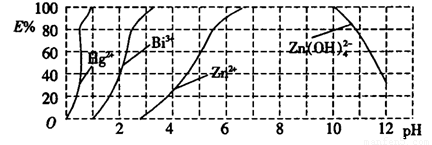
 Cu(HDz)2+2H����
Cu(HDz)2+2H����